Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
Episode 2: Improvement of decoy molecule by amino acid linkage and co-crystal structure with P450BM3
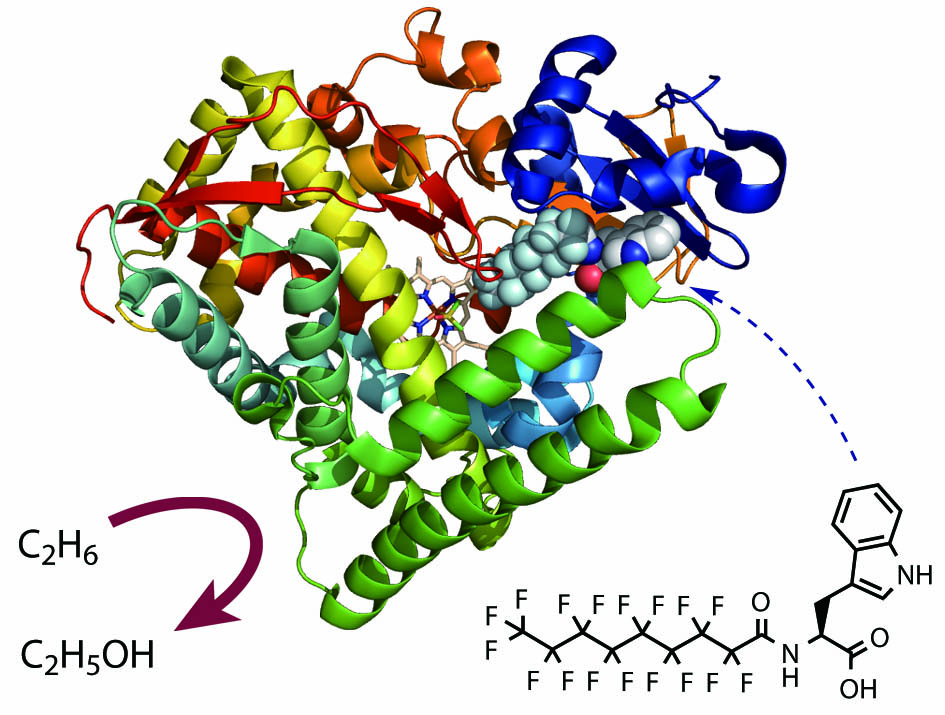
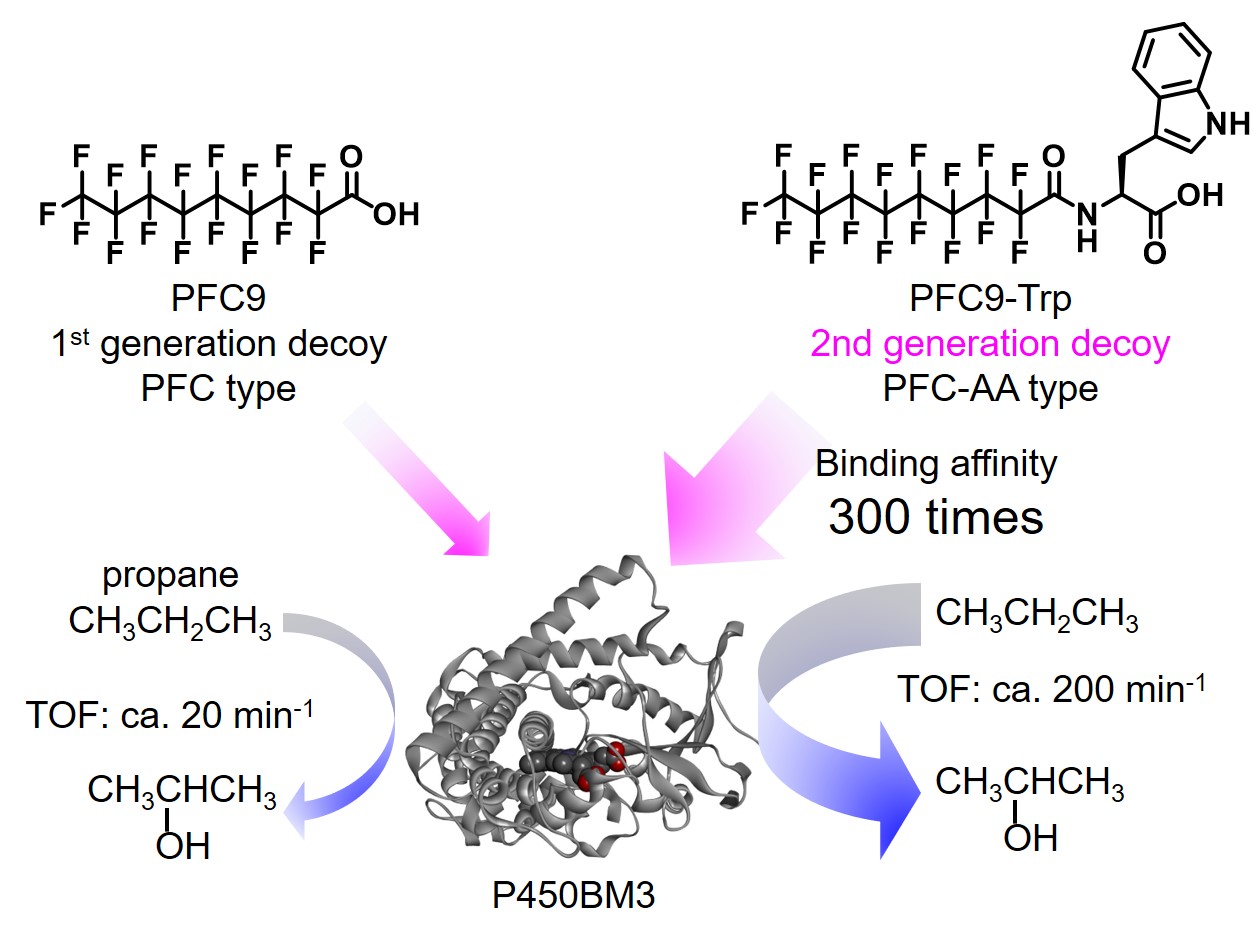
Perfluorocarboxylic acids (PFCs, 1st generation decoy) functioned as decoy molecules of P450BM3 and succeeded in hydroxylation of non-natural substrates such as propane, but the reaction rates were very slow. The cause is expected to be a low binding affinity between the decoy molecules and P450BM3.Then, once again focusing on the natural substrates, the compound (N-palmitoylglycine) in which the amino acid is linked to the fatty acid has more interaction points with P450BM3 than the simple fatty acid (ex. palmitic acid), resulting in higher binding affinity and higher hydroxylation rate.
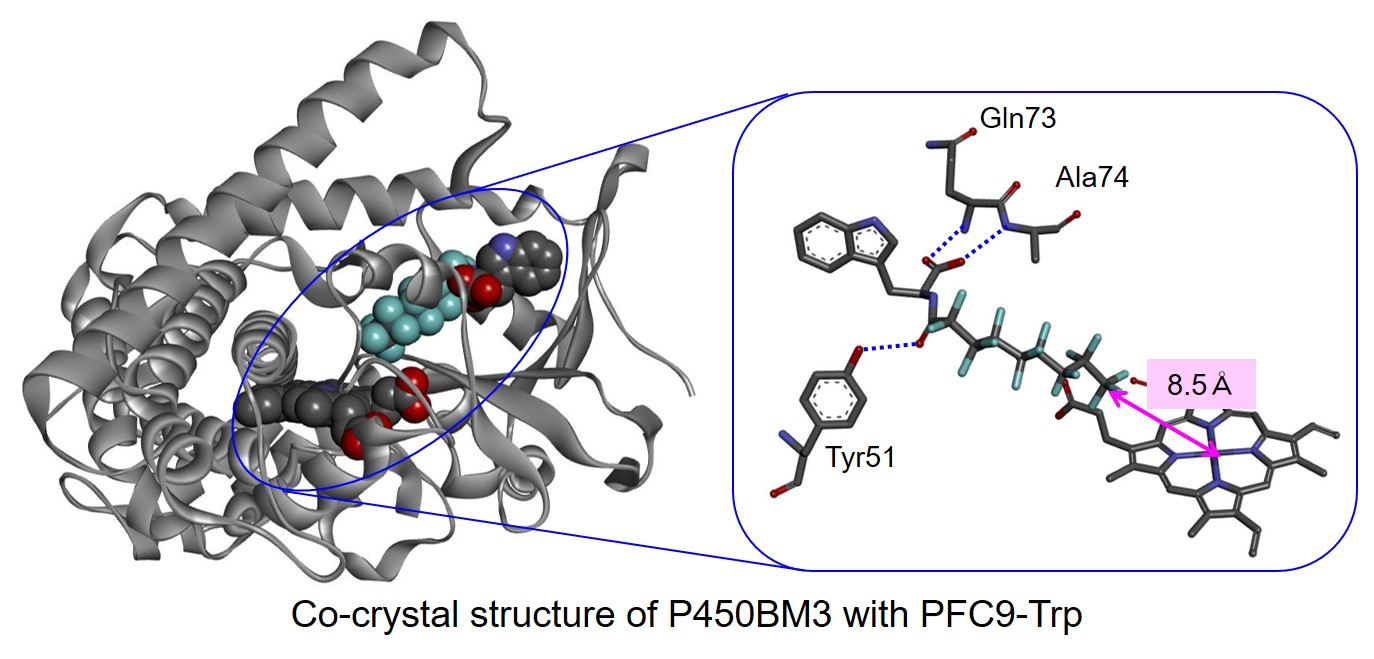
Therefore, we designed a PFC-AA type decoy molecules (2nd generation decoy) in which amino acids (AA) are linked to 1st generation decoys (PFC). PFC-AA type decoys show stronger binding to P450BM3 than PFC type decoy. For example, PFC9-Trp, which consists of PFC9 and tryptophan, binds to P450BM3 with 600 times stronger binding affinity.As expected, this strong binding affinity makes it possible for PFC-AA type decoys to greatly improve the hydroxylation activity of non-natural substrates by P450BM3, and the reaction rate of propane hydroxylation exceeded TOF 200 min-1. In addition, the strong binding affinity of 2nd generation decoys also provided important information of co-crystal structure of P450BM3 and the decoy molecules. Until this point, we tried to obtain co-crystals with P450BM3 using 1st generation decoys. But due to insufficient binding power, the obtained crystal structures of P450BM3 did not contain decoy molecules.On the other hand, the second generation decoys have strong binding affinities, so that the complexes with P450BM3 become more stably and co-crystals are obtained. In the obtained co-crystal structure of P450BM3 + PFC9-Trp, it was confirmed that Trp, which is the amino acid part of the decoy molecule, and Tyr51, Gln73, Ala74 of P450BM3 were fixed by multipoint hydrogen bonds. This structure supports the improvement of the binding affinities of PFC-AA type decoys with P450BM3 by modification.The crystal structure also reveals that there is a distance of 8.5Å between the terminus of PFC9-Trp and the heme in P450BM3, and the decoy molecule creates a space for the non-natural substrate to enter into the substrate pocket of P450BM3.
Please refer to this paper for details.
- Z. Cong, O. Shoji, C. Kasai, N. Kawakami, H. Sugimoto, Y. Shiro, Y. Watanabe, "Activation of Wild-type Cytochrome P450BM3 by the Next Generation of Decoy Molecules: Enhanced Hydroxylation of Gaseous Alkanes and Crystallographic Evidence", ACS Catalysis, 5, 150–156 (2015).
http://dx.doi.org/10.1021/cs501592f