Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
Episode 17: Directed evolution of P450BM3 mutants that can utilize microbial biosynthetic molecules as decoys and development of a self-supplying reaction system for the decoy molecules
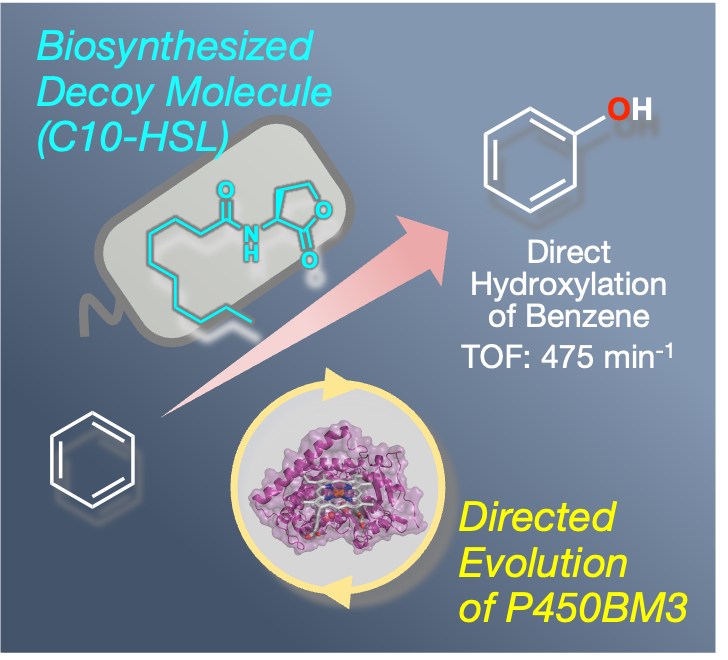
We have previously achieved highly efficient hydroxylation of unnatural substrates such as benzene by a substrate misrecognition system of P450BM3 using decoy molecules and have also succeeded in the development of a whole-cell reaction system using E. coli overexpressing P450BM3 as a reaction vessel. In this reaction system, the coenzyme NADPH required to activate P450BM3 is regenerated within E. coli, so benzene hydroxylation can be easily performed by adding unnatural substrates such as benzene and decoy molecules. However, chemical syntheses of decoy molecules in advance are required, and using organic solvents derived from fossil fuels is unavoidable. To achieve a chemical conversion method with less environmental impact, we focused on acyl-homoserine lactones, a quorum-sensing molecule that some microorganisms secrete to the outside to sense their cell density. The structure of acyl-homoserine lactones is similar to acylamino acids (e.g., CnPhe), a fluorine-free third-generation decoy molecule. Therefore, we aimed to develop a self-sufficient reaction system by introducing acyl-homoserine lactone synthase into E. coli, producing decoy molecules intracellularly and avoiding the addition of chemically synthesized decoy molecules.
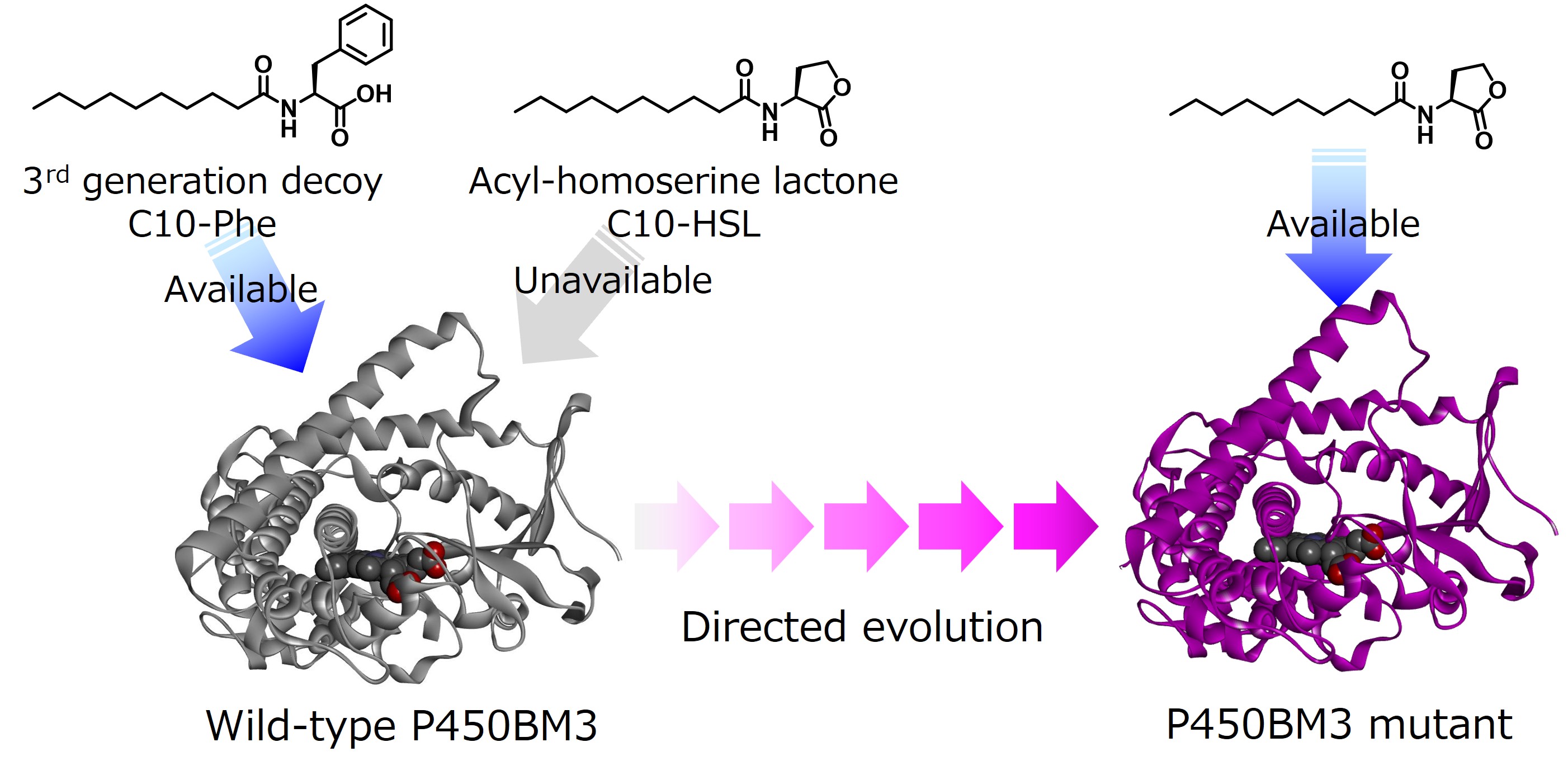
However, acyl-homoserine lactone does not contain a carboxyl group, which is essential for the decoy molecule of P450BM3. It is not recognized as a decoy molecule by wild-type P450BM3, and benzene hydroxylation cannot proceed. Therefore, we used directed evolution, the most versatile and successful method for modifying the enzyme's function. In this method, a library containing many mutants in which the amino acids constituting the target enzyme have been replaced with other ones is created. Among them, mutants with a high conversion efficiency of the target non-natural substrate are searched for. The obtained mutant is used as the next parent, and mutations are introduced, gradually evolving the enzyme mutant into one suitable for the target reaction. This technique was the subject of the Nobel Prize in Chemistry in 2018. We used directed evolution to evolve P450BM3 mutants that respond to acyl homoserine lactone as a decoy molecule rather than adapt it to the substrate. After five generations of evolution, the V-19A14 mutant, which contained eight mutations, strongly recognized acyl-homoserine lactone as a decoy molecule and recorded a maximum benzene hydroxylation conversion rate of 41% in the whole-cell reaction.
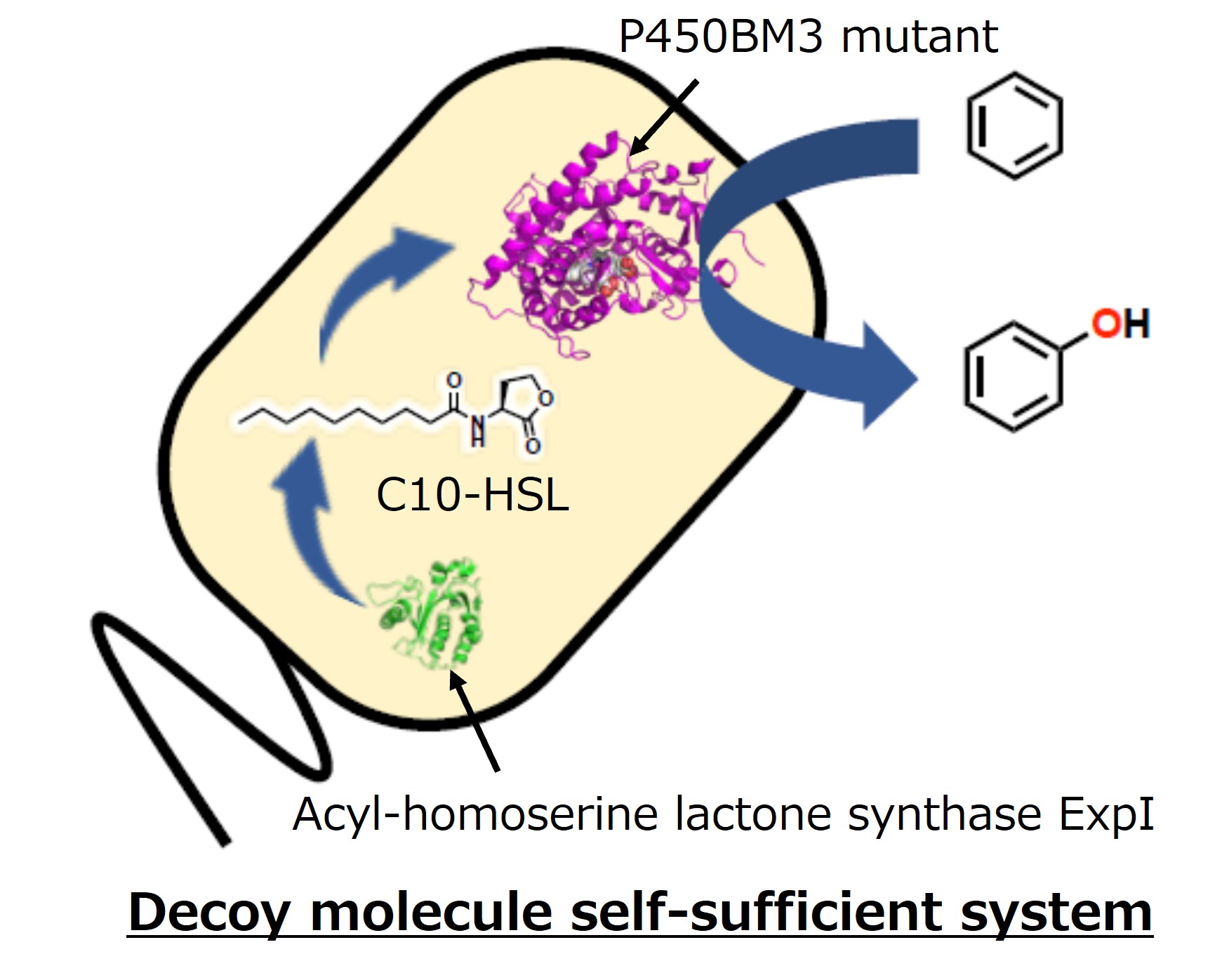
Furthermore, the gene for acyl-homoserine lactone synthase ExpI from Pectobacterium carotovorum was introduced to a plasmid and transformed into E. coli to create E. coli expressing the P450BM3 V-19A14 mutant and ExpI. In this E. coli, ExpI biosynthesizes acyl-homoserine lactone within the cell, and P450BM3 V-19A14 uses it as a decoy molecule, enabling the conversion of benzene to phenol without the addition of an external decoy molecule. This achievement is expected to be applied to chemical conversion with low environmental impact as a decoy molecule self-supplying reaction system.
Please refer to this paper for details.
- Y. Yokoyama, S. Ariyasu, M. Karasawa, C. Kasai, Y. Aiba, H. Sugimoto, O. Shoji, "Bacterial acyl homoserine lactones triggered non-native substrate hydroxylation catalysed by directed-evolution-derived cytochrome P450BM3 mutants" , ChemCatChem, 16, (2024) e202401641
https://doi.org/10.1002/cctc.202401641