Research Introduction
Artificial metalloproteins based on heme acquisition protein HasA
Episode 2: Conjugation of HasA from Pseudomonas aeruginosa with 5,15-diphenylporphyrin iron complexes and its analogs
The heme acquisition protein HasA secreted by Pseudomonas aeruginosa can capture various iron complexes, including iron salophen, iron mesoporphyrin IX, and iron phthalocyanine. In particular, HasA that captured iron phthalocyanine showed potent growth inhibition against Pseudomonas aeruginosa.
On the other hand, porphyrin complexes have attracted attention as functional molecules in various fields such as coordination chemistry, photochemistry, electrochemistry, and catalytic chemistry due to their characteristic planar structure and electron conjugation system, and a vast number of complexes have been synthesized and evaluated to date. If HasA can capture porphyrin complexes, which are significantly different from heme, their application potential is expected to be greatly expanded.
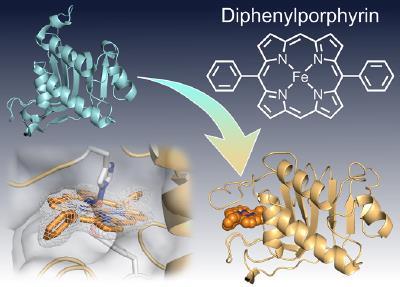
Therefore, we focused on 5,15-diphenylporphyrin (DPP) among many synthetic porphyrin complexes. DPP can be synthesized in good yield by simply reacting dipyrromethane with benzaldehyde derivatives in the presence of an acid catalyst. DPP derivatives with different meso-substituents can be easily prepared. However, there were no examples of conjugation of DPP with natural proteins.
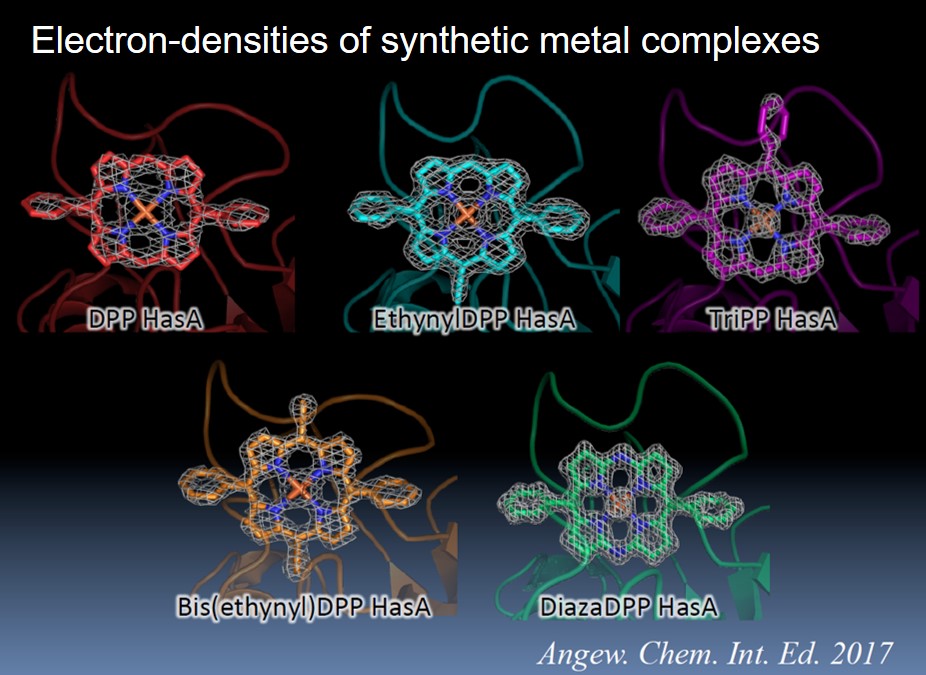
In cooperation with Professor Shinokubo’s (Nagoya University) group, we synthesized various iron DPP complexes and conjugated them with HasA derived from Pseudomonas aeruginosa. In addition to the simplest DPP, many iron DPP complexes, including TriPP with three phenyl groups, DPP with one or two ethynyl groups, and DiazaDPP with a skeleton of nitrogen at the meso position, were able to be conjugated to HasA. We also succeeded in analyzing the crystal structure of each iron DPP complex-HasA complex and found that the two phenyl groups at the meso positions were exposed to the solvent to avoid steric hindrance with HasA. The porphyrin ring in HasA was rotated about 45 degrees compared with the heme. Interestingly, when the growth inhibition abilities of the various iron DPP complexes-HasA complexes were evaluated, it was found that the growth inhibition ability differed greatly depending on the structure of the complexes. However, there was almost no difference in the three-dimensional structure of HasA in the crystals. Although it is not clear which factors of the complex structures highly affect the growth inhibition of Pseudomonas aeruginosa, this study demonstrated that HasA is capable of capturing synthetic porphyrin complexes, providing a stepping stone for the development of the HasA-synthetic porphyrin complex system.
Please refer to this paper for details.
- H. Uehara, Y. Shisaka, T. Nishimura, H. Sugimoto, Y. Shiro, Y. Miyake, H. Shinokubo, Y. Watanabe, O. Shoji, " Structures of the Heme Acquisition Protein HasA with Iron(III)-5,15-Diphenylporphyrin and Derivatives Thereof as an Artificial Prosthetic Group", Angew. Chem. Int. Ed., 56, 15297–15283 (2017).
http://dx.doi.org/10.1002/anie.201707212