Research Introduction
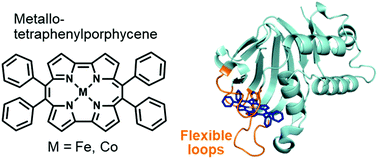
Artificial metalloproteins based on heme acquisition protein HasA
Episode 3: Complexation of HasA from Pseudomonas aeruginosa with 9,10,19,20-tetraphenylporphycene complex
The heme acquisition protein HasA of Pseudomonas aeruginosa can capture various iron-DPP complexes, and analysis of the crystal structure showed that the two bulky phenyl groups at meso positions are positioned to avoid contact with HasA. From this crystal structure, we speculated that even bulkier scaffolds can be captured by HasA if they are located at the meso-positioned phenyl groups of DPP.
Therefore, in a joint research project with Prof. Hisaeda's group at Kyushu University, we attempted to combine HasA with iron and cobalt complexes of 9,10,19,20-tetraphenylporphycene (Ph4Pc). Ph4Pc is a porphyrin-like skeleton whose synthesis method on a gram scale has been established in Prof. Hisaeda's group. This complex has attracted attention in photochemistry and catalytic chemistry due to its physical properties that are significantly different from those of porphyrins, such as strong visible light absorption at long wavelengths.
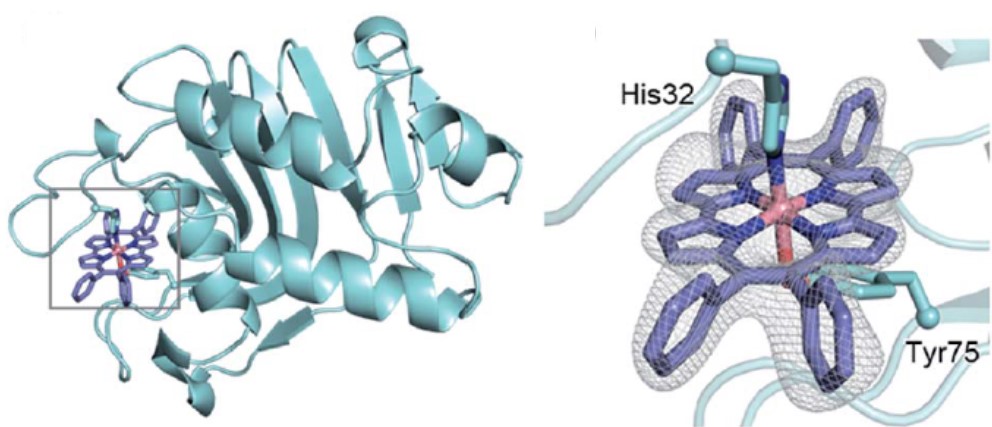
We complexed Fe-Ph4Pc and Co-Ph4Pc provided by Prof. Hisaeda’s group with Pseudomonas aeruginosa HasA using the conventional method, and as expected, HasA could stably capture the Ph4Pc complex. Furthermore, we analyzed the crystal structure of the Co-Ph4Pc-HasA complex, and the four phenyl groups of Ph4Pc were exposed to the solvent in an orientation that avoided contact with HasA. Compared with the structure of holo-HasA that captured heme, there was a shift in the loop around the heme-binding site. Still, it is speculated that this flexible loop allows HasA to complex even bulky synthetic metal complexes such as Ph4Pc, which are very different from heme. In addition, Fe or Co-Ph4Pc-HasA also showed the ability to inhibit the growth of Pseudomonas aeruginosa, and although the only difference was the central metal, Fe-Ph4Pc had a higher growth inhibition ability than Co-Ph4Pc, and it was revealed that in addition to the complex structure, the type of metal also affects the growth inhibition ability.
Please refer to this paper for details.
- E. Sakakibara, Y. Shisaka, H. Onoda, D. Koga, N. Xu, T. Ono, Y. Hisaeda, H. Sugimoto, Y. Shiro, Y. Watanabe, O. Shoji, "Highly malleable haem-binding site of the haemoprotein HasA permits stable accommodation of bulky tetraphenylporphycenes", RSC Adv., 9, 18697–18702 (2019).
https://doi.org/10.1039/C9RA02872B