Research Introduction
Artificial metalloproteins based on heme acquisition protein HasA
Episode 7: Conjugation of Phenanthroline-linked Porphyrin with HasA from Pseudomonas fluorescens and Construction of a Bevel Gear Dimer by Metal Coordination
We have succeeded in conjugating various synthetic metal complexes, such as salophen, phthalocyanine, porphycene, and tetraphenylporphyrin (TPP), to the heme acquisition protein HasA derived mainly from Pseudomonas aeruginosa. Among these, TPP is a porphyrin derivative that is easy to synthesize and a highly scalable metal complex. Still, its HasA conjugates have the disadvantage that they can only exist stably under alkaline conditions and are unstable at near-neutral pH when combined with Pseudomonas aeruginosa HasA (HasAp).
A homology search for HasAp led to the discovery of HasA (HasApf5) from Pseudomonas protegens PF-5. Surprisingly, despite its high sequence homology with HasAp, HasApf5 could maintain the Fe-TPP complex even near neutral pH. Crystal structure analysis revealed the presence of hydrogen bonds not seen in HasAp, suggesting that these contribute to stability.
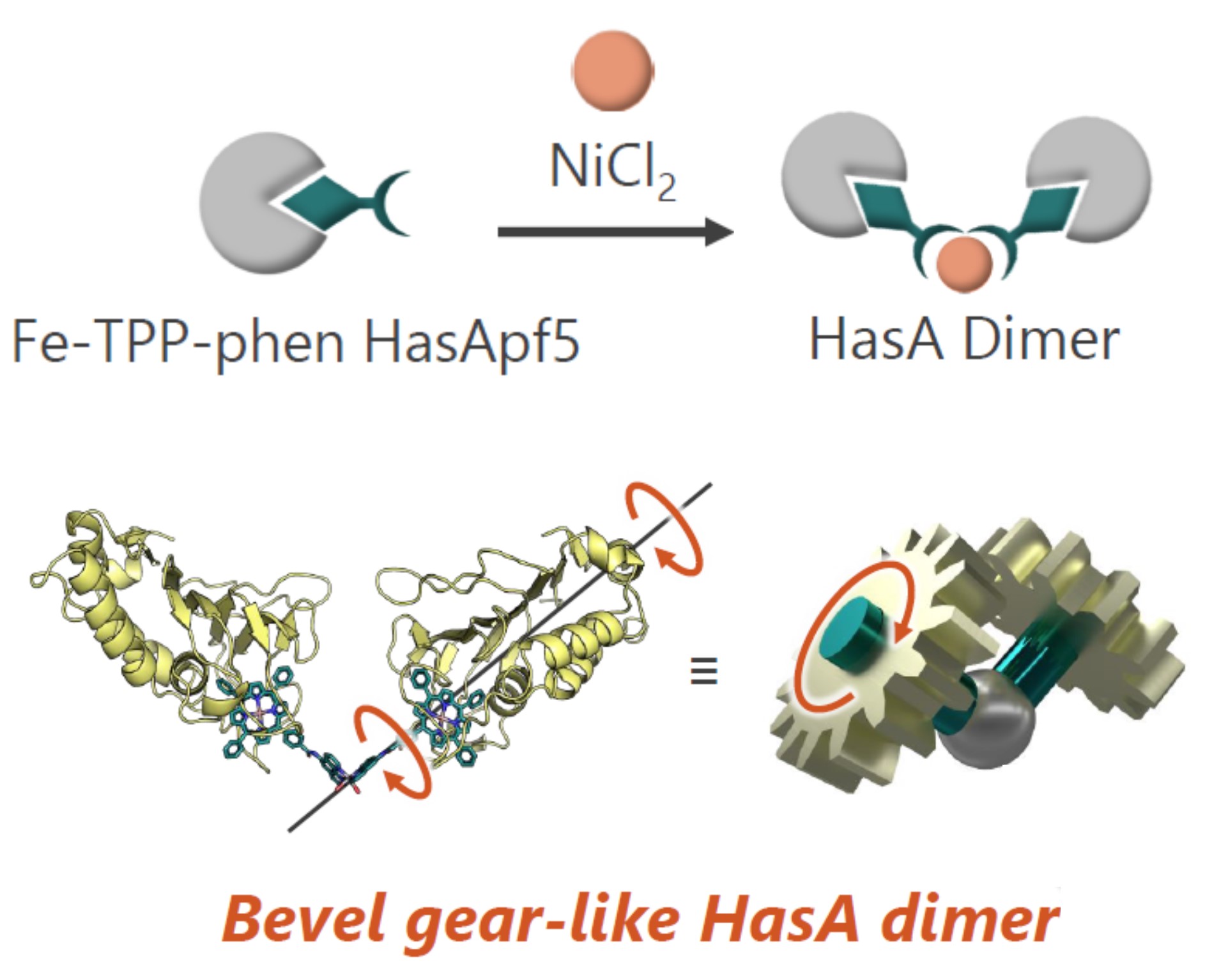
Taking advantage of the excellent complex-capturing ability of HasApf5, we synthesized Fe-TPP-phen, in which phenanthroline, a well-known bidentate ligand, was linked to one phenyl group of TPP and complexed it with HasApf5. We clarified that when Ni2+ ions were added to this, Ni ions were coordinated at the phen moiety of Fe-TPP-phen-HasApf5, forming a HasApf5 dimer. To obtain structural information about this dimer, we analyzed it by small-angle X-ray scattering (SAXS) and found that the two HasApf5s in the dimer form a V-shape with the Ni-phen complex at the apex. SAXS results suggest that HasApf5 can rotate while maintaining this arrangement, and it is speculated that the dimer forms a bevel gear-shaped dimer, which is rare for an artificial protein dimer.
Please refer to this paper for details.
- H. Inaba, Y. Shisaka, S. Ariyasu, E. Sakakibara, G. Ueda, Y. Aiba, N. Shimizu, H. Sugimoto, O. Shoji, "Heme-Substituted Protein Assembly Bridged by Synthetic Porphyrin: Achieving Controlled Configuration while Maintaining Rotational Freedom" , RSC Adv.,14, 8829–8836 (2024).
https://doi.org/10.1039/D4RA01042F