Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
Episode 3: Improvement of decoy molecule by amino acid linkage and co-crystal structure with P450BM3
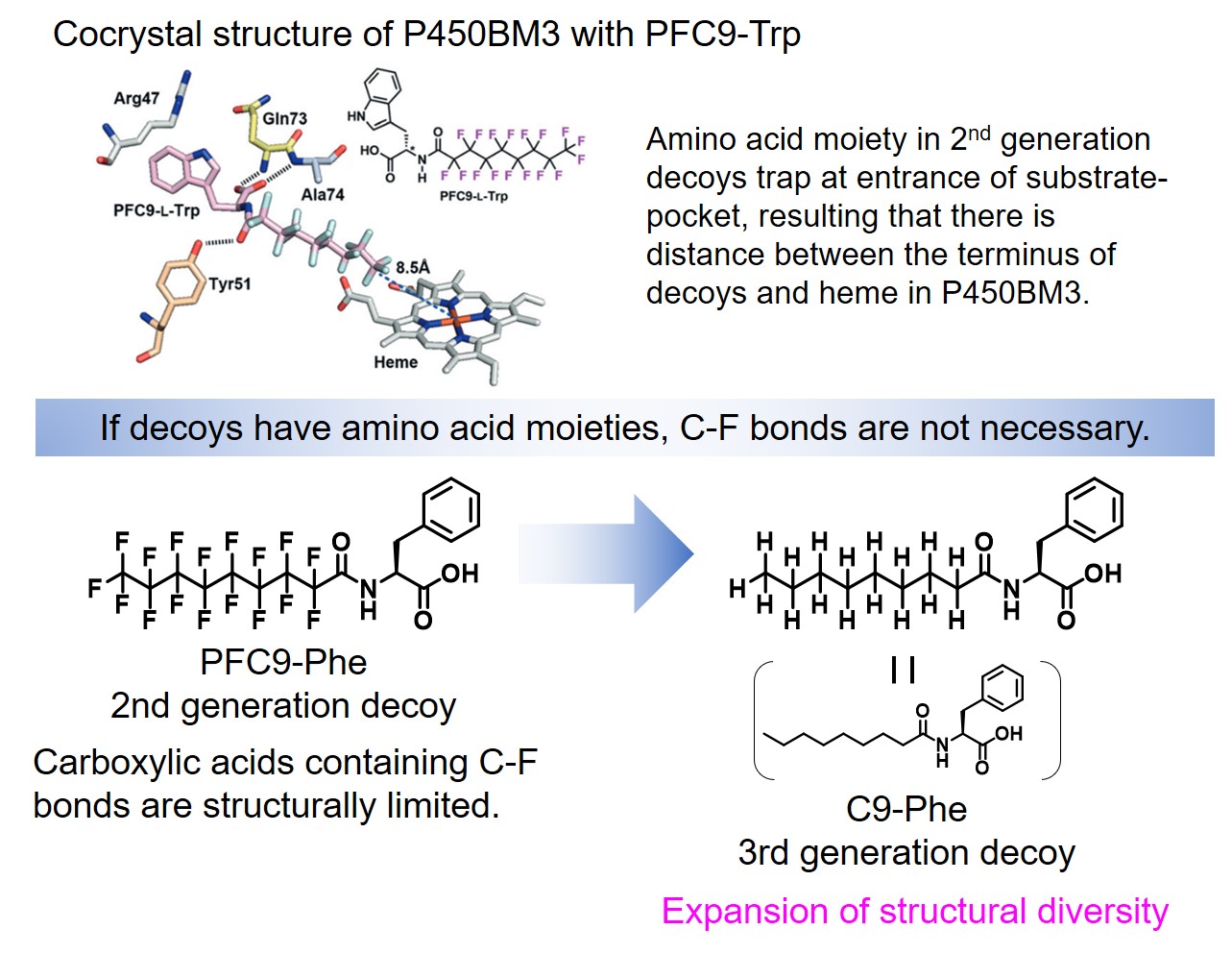
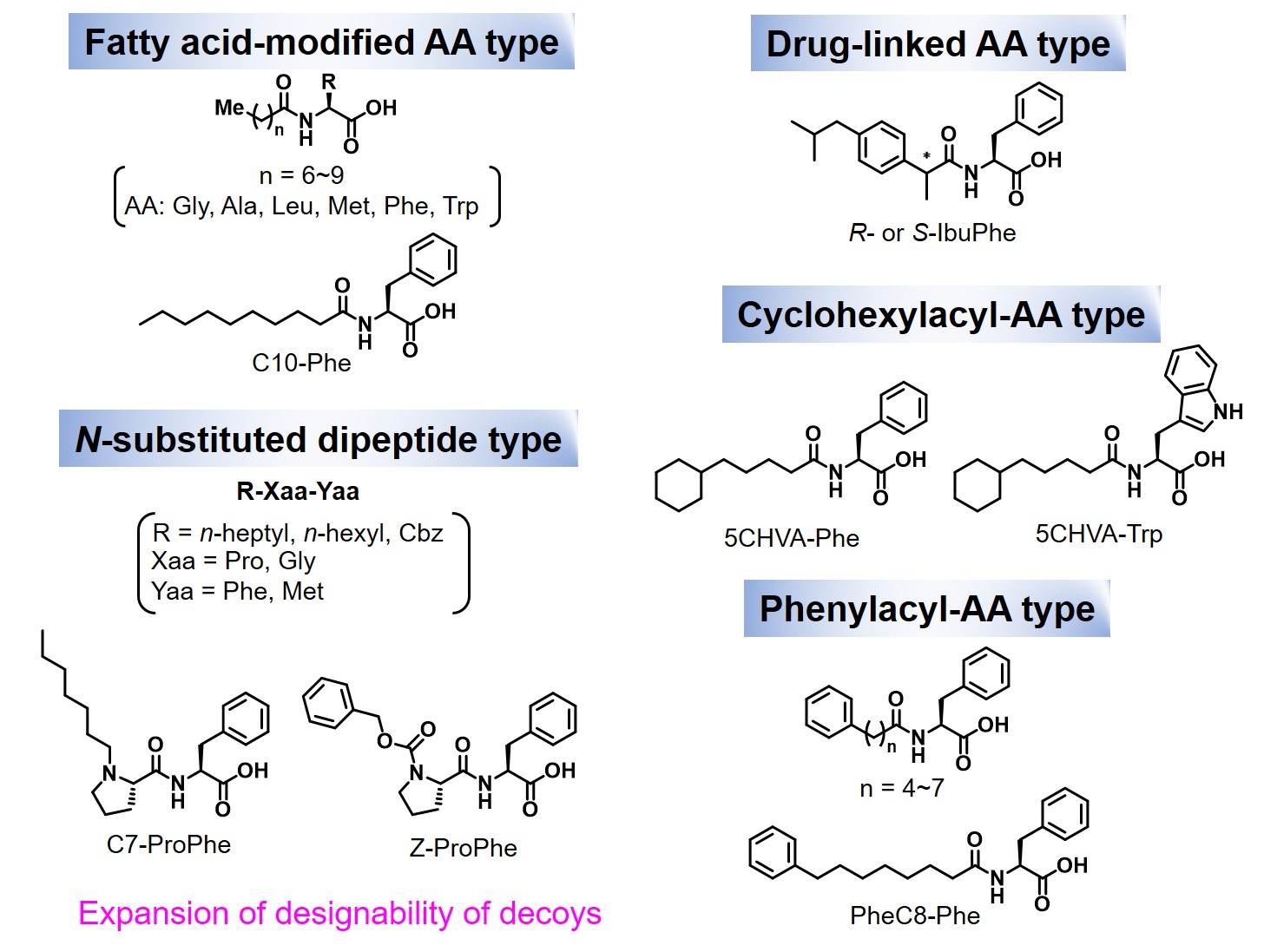
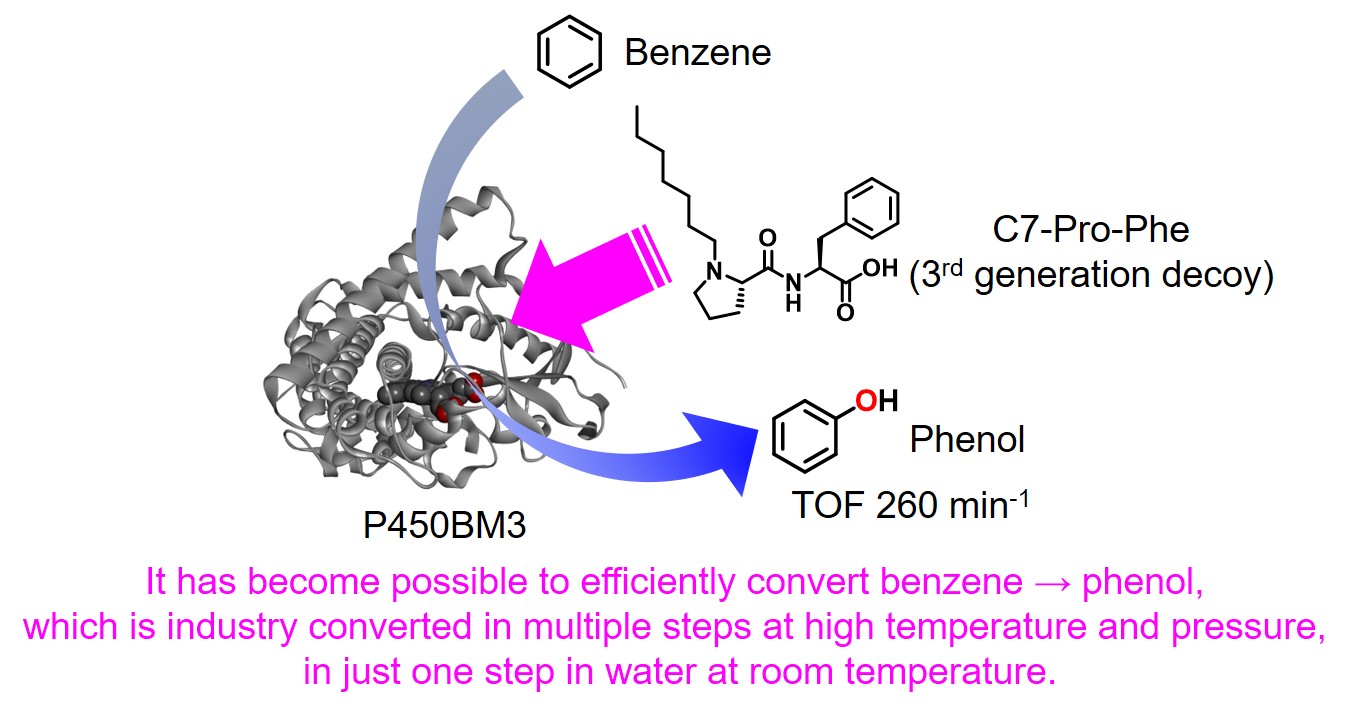
Structural analyses of cocrystal of P450BM3 with the 2nd generation decoy (PFC-AA type) led to a significant expansion of the structural diversity of decoy molecules. According to the crystal structure, the PFC-AA type decoy is strongly fixed at the amino acid moiety at the substrate-pocket entrance of P450BM3, so the terminus of the decoy molecule is clearly located at the position that has some distance to the active center heme.The C-F bond was introduced to prevent hydroxylation of the decoy molecule itself, but the hypothesis is "If the decoy tip does not contact heme due to amino acid modification, isn't it necessary to be C-F bond?" As a result, FA (Fatty acid)-AA type decoys(3rd generation decoy such as C9Phe and C10Phe), in which amino acids are modified to medium chain fatty acids without C-F bond, were developed. In hydroxylation of non-natural substrates such as benzene by P450BM3 in the presence of 3rd generation decoys, these decoy molecules without C-F bonds were not hydroxylated and the non-natural substrates were hydroxylated. Surprisingly, by modifying amino acid moieties, not only linear carboxylic acids such as fatty acids, but also complicated carboxylic acids that differ greatly from natural substrates, such as ibuprofen can also function as a decoy molecule. Furthermore, dipeptides modified with alkyl chains such as C7-Pro-Phe as shown in the figure were also available as decoy molecules. In particular, in C7-Pro-Phe, the direct conversion from benzene to phenol proceeded efficiently, and about TOF 260 min-1 were observed. Until that time, since it was considered that C-F bonds were essential for decoy molecules, carboxylic acids having inactive C-F bonds were not easy to be chemically converted and its structure was limited, resulting that the types of decoy molecules were greatly limited. However, because it turns out that C-F bonds are not essential for decoy molecules, a large number of commercially available products and natural carboxylic acids can be considered as candidates for decoy molecules by simple modification with amino acids, resulting that structural diversity of decoys was greatly expanded. The decoy molecules partially occupy the substrate pocket of P450BM3 and have a role to construct a reaction space suitable for non-natural substrates. By designing decoy molecules that can rearrange to optimal reaction spaces for various non-natural substrates, it is expected that more types of non-natural substrates will be hydroxylated with P450BM3 than ever before.
Please refer to this paper for details.
- O. Shoji, S.Yanagisawa, J. Kyle Stanfield, K. Suzuki, Z. Cong, H. Sugimoto, Y. Shiro, Y. Watanabe , " Direct Hydroxylation of Benzene to Phenol by Cytochrome P450BM3 Triggered by Amino Acid Derivatives ", Angew. Chem. Int. Ed., 56, 10324-10329 (2017). The frontispiece picture of issue 35.
http://dx.doi.org/10.1002/anie.201703461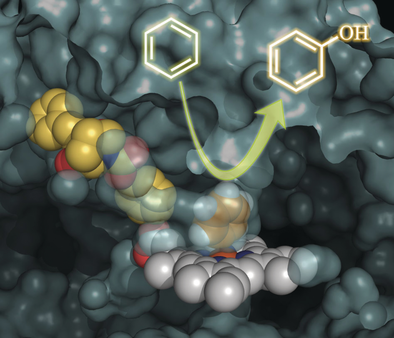
Chapter 2 Episode 4: Control of stereoselectivity in hydroxylation by decoy molecules


