Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
Episode 12: Investigation of the reaction properties of manganese-centered heme-substituted P450BM3
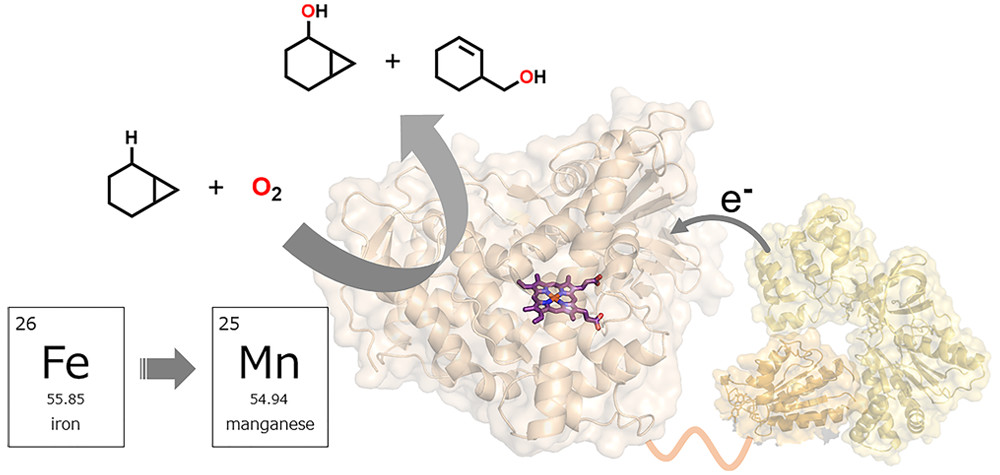
Previously, we succeeded in creating several heme-substituted P450BM3s, including manganese, by expressing the heme domain and the reductive domain separately and replacing the heme (iron complex) in the heme domain with a heme analog with a different central metal (Research Introduction to P450BM3, Part 5). In addition, P450BM3 with manganese as the central metal can activate oxygen molecules and hydroxylate non-natural substrates such as propane in the presence of decoy molecules, just like natural iron-containing P450BM3. Although this is a rare example in manganese-containing metalloenzymes, the catalytic efficiency is lower than that of natural iron-centered P450BM3s, and the reaction mechanism had not been elucidated.
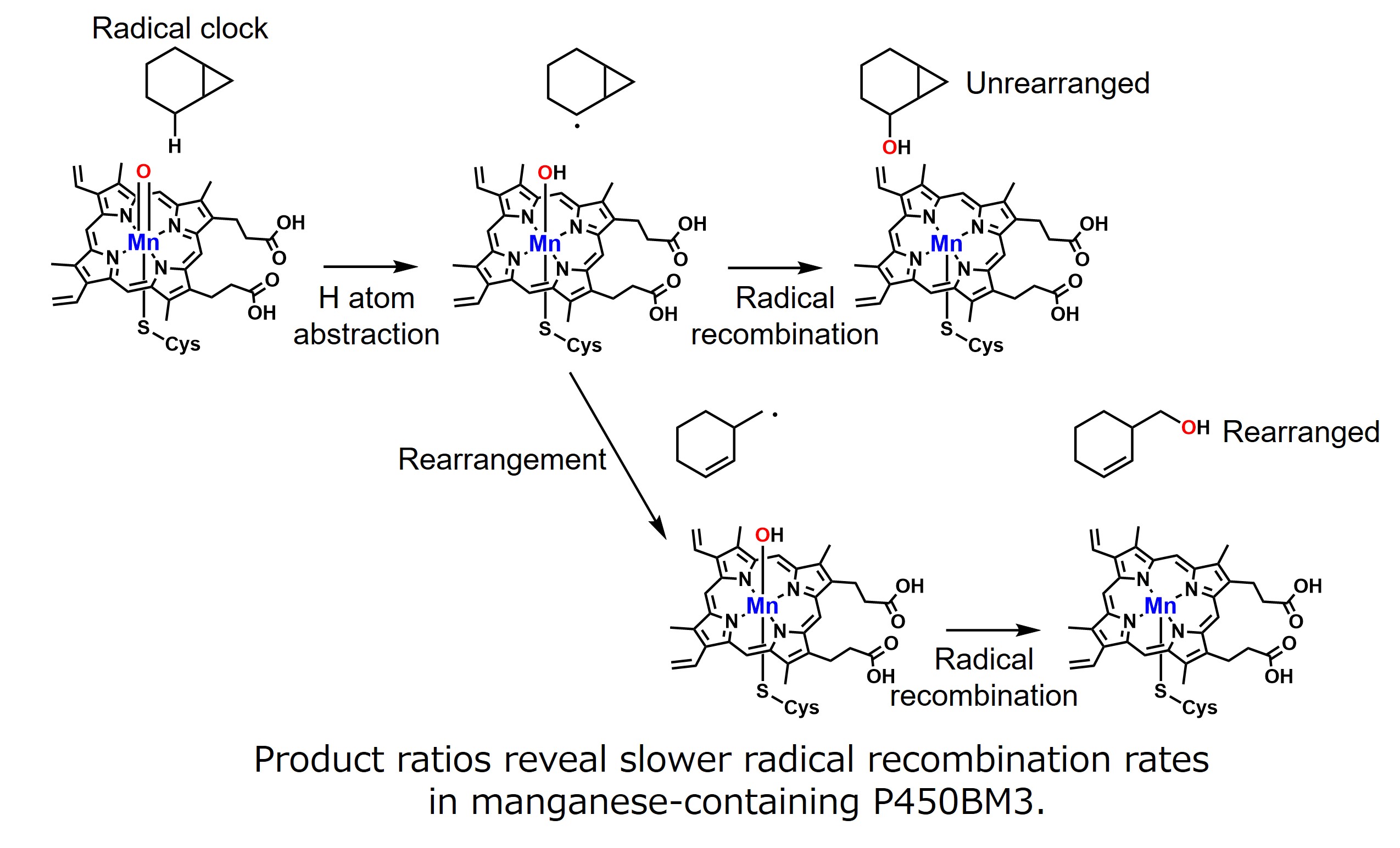
First, we performed the hydroxylation of cyclohexane with 16O2 (a general oxygen molecule) and 18O2 in the presence of decoy molecules using manganese-containing P450BM3. We confirmed that 18O was incorporated into the product cyclohexanol, indicating that oxygen molecules are used as the oxygen source. We also found that the I401P mutation, which replaces Ile400, located next to Cys400, the axial ligand for the heme of P450BM3, with Pro, is useful for improving the catalytic efficiency of manganese-containing P450BM3. To understand the reaction properties of manganese-containing P450BM3, we performed a hydroxylation reaction using a known radical clock molecule (1-methyl-2-phenylcyclopropane, Norcarane) as a substrate. We showed that the reaction mechanism was hydrogen atom abstraction/radical recombination, similar to natural iron-centered P450BM3. We also estimated the speed of radical recombination of oxidizing active species from the ratio of the two products and found that manganese-containing P450BM3 has slower radical recombination than natural iron-centered P450BM3, revealing that it has different reaction properties from natural iron-centered P450BM3.
Please refer to this paper for details.
- K. Omura, Y. Aiba, K. Suzuki, S. Ariyasu, H. Sugimoto, O. Shoji "A P450 Harboring Manganese Protoporphyrin IX Generates a Manganese Analogue of Compound I by Activating Dioxygen" , ACS Catal., 12, (2022) 11108-11117.
https://doi.org/10.1021/acscatal.2c01345