Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
Episode 13: Crystal structure analysis of Compound I mimicking P450BM3 using oxo molybdenum porphyrin
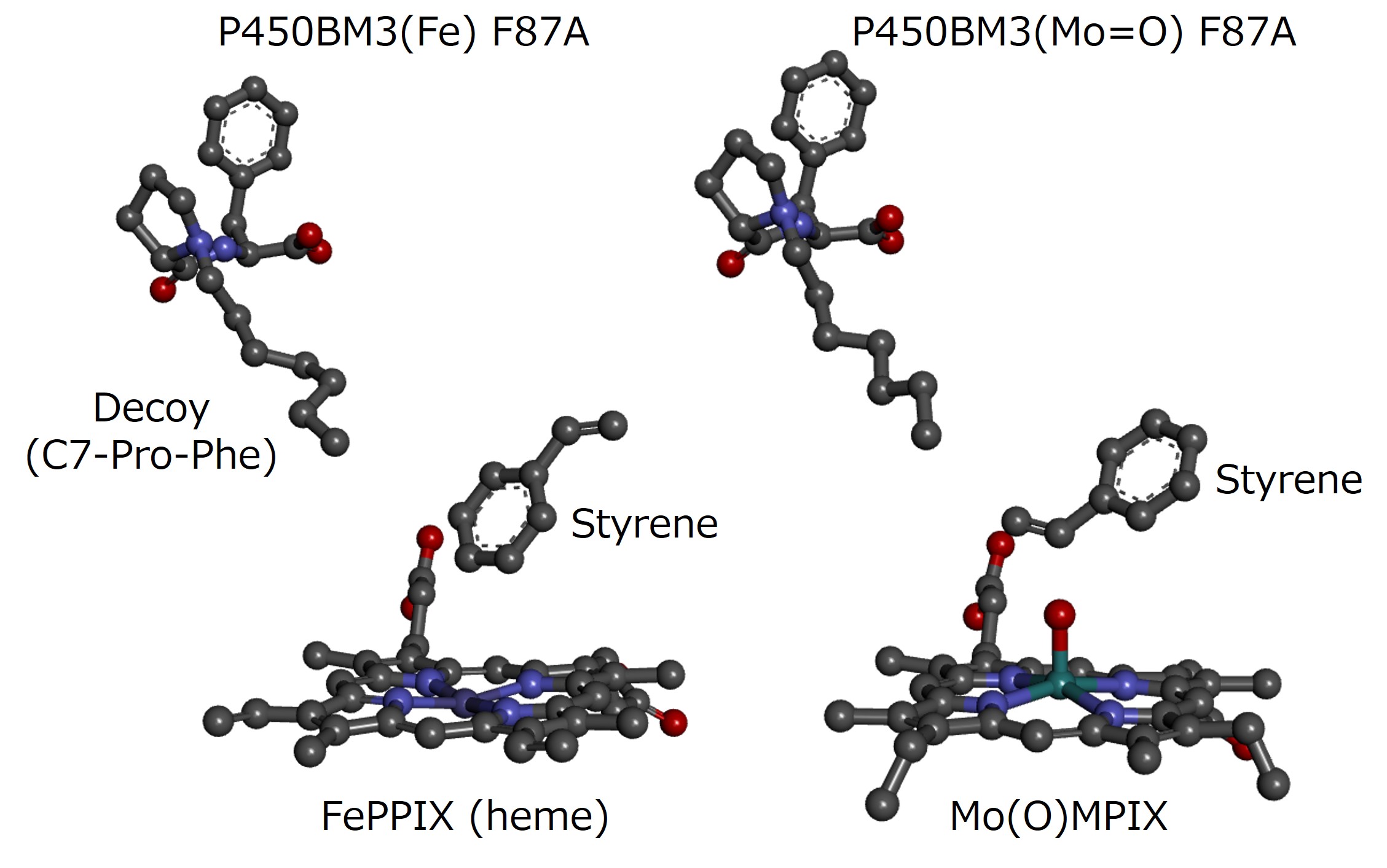
We have developed a crystallization-inducing decoy molecule (Episode 8, "Rapid crystallization of P450BM3 by crystallization-inducing decoy molecule"), which has made it much easier to obtain structural information on P450BM3 with the decoy molecules. Furthermore, we have succeeded in obtaining the three-dimensional structure of the ternary complex of P450BM3 + decoy molecule + substrate by soaking the substrates to the obtained crystal, which incorporates the substrate molecule into the P450BM3 + decoy molecule crystal. This made it possible to confirm how the substrate and decoy molecule are oriented in the substrate pocket of P450BM3, which contributes to the structural discussion on the substrate misrecognition system, but for some substrates, the results obtained were inconsistent with the experimental results. For example, when styrene is added as a substrate to P450BM3 with decoy molecules, epoxidation of the vinyl group proceeds instead of hydroxylation of the aromatic ring, but in the cocrystal of P450BM3 + decoy + styrene, the styrene is oriented with the aromatic ring facing the iron of P450BM3, which is different from the selectivity of the enzymatic reaction. In response to this, we believe that the crystal structure observed so far is close to the resting state of P450BM3 and does not express the structure of the reactive intermediate during the oxidation reaction.
Therefore, we used the heme substitution technology of P450BM3 that we have developed so far (Episode 5, "Reconstitution of P450BM3 for incorporation of different metal centers") to construct oxo molybdenum P450BM3 with oxo molybdenum mesoporphyrin IX at the center instead of heme (iron protoporphyrin IX). Oxo molybdenum species are pentavalent molybdenum species but are very stable and have poor oxidative activity. However, their structure is similar to compound I, which is the reactive species of heme enzymes, and we thought that they could be considered as a stable compound I model. We soaked the crystals of oxo molybdenum P450BM3 and decoy molecules, created by adding crystallization-inducing decoy molecules, in a styrene-saturated buffer solution. We analyzed the crystal structure of the obtained ternary complex crystals. The styrene was bound with the vinyl group oriented toward the oxo molybdenum. In addition, the P450BM3 F87A mutant selectively gave the R-form in styrene epoxidation. In contrast, the F87V mutant selectively gave the S-form. Still, in the crystal structures of the F87A and F87V oxo molybdenum P450BM3, the vinyl group of styrene and the oxo molybdenum were close to each other in the orientation that gave the R-form and the S-form, respectively, which was consistent with the stereoselectivity of the enzyme reaction. This suggests that the oxo molybdenum P450BM3 may provide similar structural information to the Compound I of P450BM3, and this result is expected to significantly contribute to elucidating the mechanism of P450 in the future.
Please refer to this paper for details.
- K. Suzuki, J. K. Stanfield, K. Omura, Y. Shisaka, S. Ariyasu, C. Kasai, Y. Aiba, H. Sugimoto, O. Shoji "A Compound I Mimic Reveals the Transient Active Species of a Cytochrome P450 Enzyme: Insight into the Stereoselectivity of P450-Catalysed Oxidations" , Angew. Chem. Int. Ed., 62, (2023) e202215706
https://doi.org/10.1002/anie.202215706

Chapter 2 Episode 14: Application of decoy molecules to CYP102A5 and A7 in subfamily of P450BM3


