Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
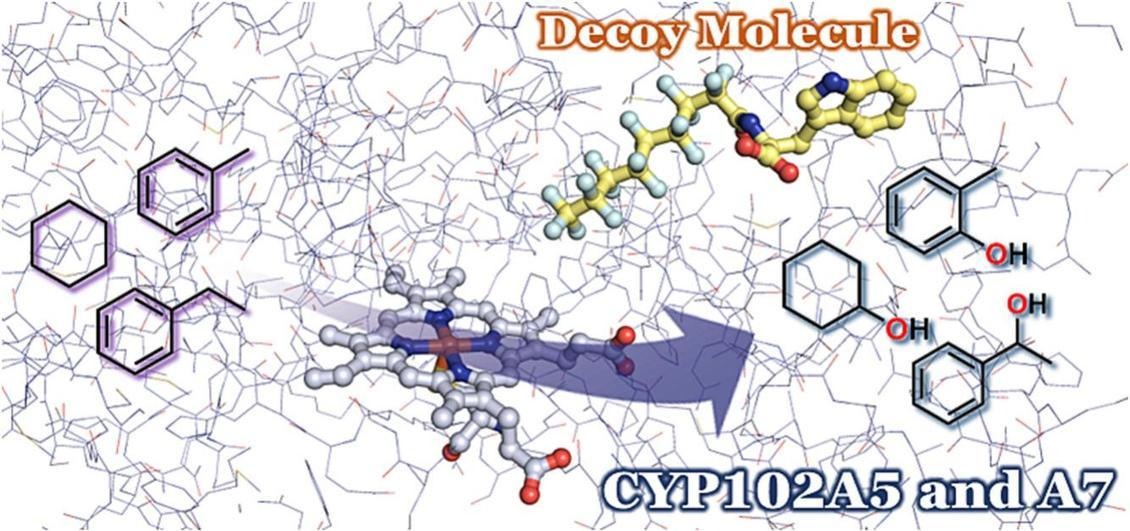
Episode 14: Application of decoy molecules to CYP102A5 and A7 in subfamily of P450BM3
Since we found that medium-chain fatty acids function as decoy molecules (Chapter 1: Functional modulation of hydrogen peroxide-driven P450 using decoy molecules), we have applied decoy molecules to the long-chain fatty acid P450BM3 (classification name CYP102A1) from Priestia megaterium (formerly Bacillus megaterium), which is a self-sufficient P450 and have succeeded in directly hydroxylating benzene, propane, and ethane. Although several P450s have been reported in the CYP102 subfamily to which P450BM3 belongs, such as CYP102A5 from Bacillus cereus and CYP102A7 from Bacillus licheniformis, there have been no examples of catalytic applications using P450s other than P450BM3 (CYP102A1) by the substrate-misrecognition system.
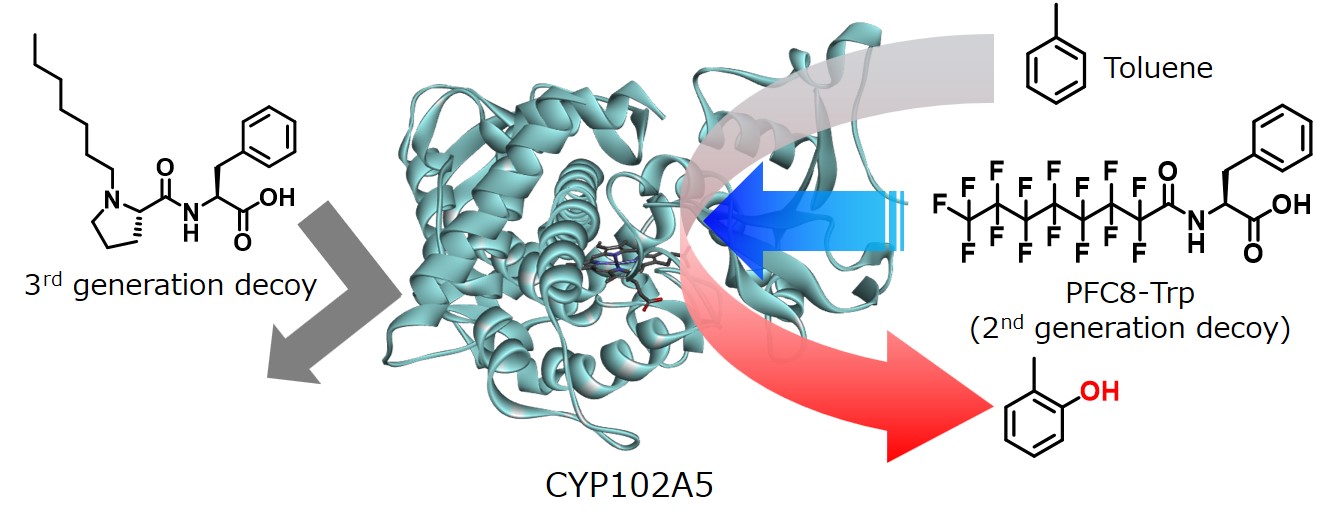
In order to broaden the scope of application of the substrate misrecognition system using decoy molecules, we focused on CYP102A5 and CYP102A7, which belong to the same CYP102 subfamily as P450BM3, and investigated the response-ability of decoy molecules. As a result, although they belong to the same CYP102 subfamily, CYP102A5 and CYP102A7 showed completely different decoy molecule response-ability from P450BM3 (CYP102A1), and CYP102A5 responded almost only to second-generation decoy molecules (amino acid-modified PFC) (Episode 2 "Improvement of decoy molecule by amino acid linkage and co-crystal structure with P450BM3"), and it was revealed that it particularly prefers tryptophan (Trp), which has the largest side chain in natural amino acids. Surprisingly, it hardly responded to the third-generation decoy molecules (Episode 3: "Expansion of structural diversity of decoy molecules by escape from fluorine atoms"), which are non-fluorine-containing decoys that can strongly induce the enzymatic function of P450BM3. On the other hand, CYP102A7 was also completely different from P450BM3 and CYP102A5 and responded strongly to the decoy molecules containing alanine (Ala) that has a small side chain among the same second-generation decoy molecules. However, neither CYP102A5 nor CYP102A7 exceeded the activity of P450BM3. Because the decoy molecules tested this time were originally developed for P450BM3, further activity improvement can be expected by developing decoy molecules for CYP102A5 and CYP102A7 in the future. In addition, we also succeeded in obtaining the crystal structure of CYP102A5, and we can expect development into the molecular design of decoy molecules based on the structure.
Please refer to this paper for details.
- J. K. Stanfield, H. Onoda, S. Ariyasu, C. Kasai, E. M. Burfoot, H. Sugimoto, O. Shoji "Investigating the applicability of the CYP102A1-decoy-molecule system to other members of the CYP102A subfamily" , J. Inorg. Biochem., 245, (2023) 112235
https://doi.org/10.1016/j.jinorgbio.2023.112235