Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
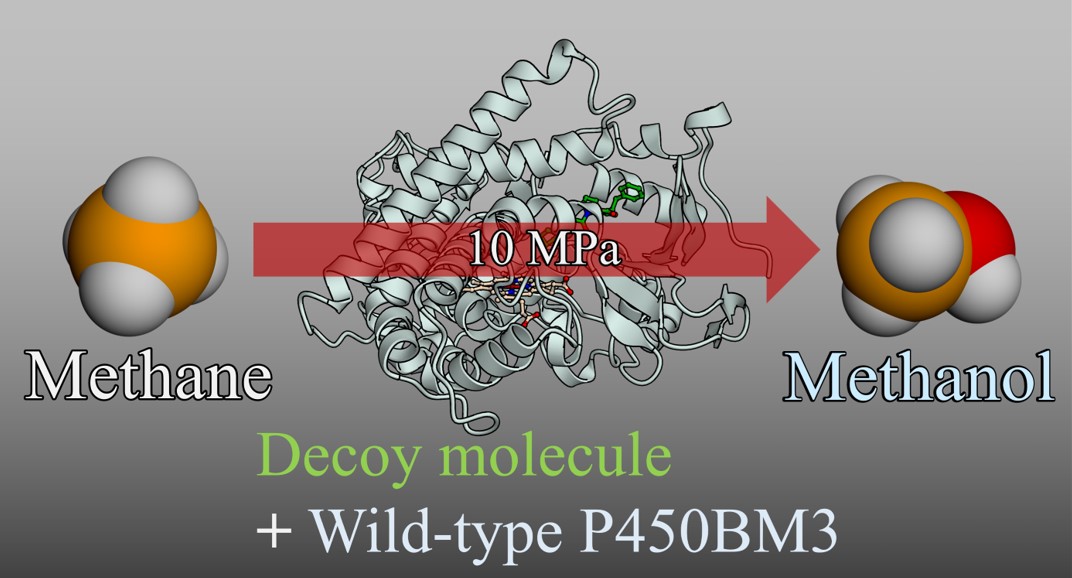
Episode 15: Catalytic methane hydroxylation by wild-type P450BM3 using chemically evolved decoy molecules
We have developed methodologies for altering enzymatic properties in long-chain fatty acid hydroxylase P450BM3 by adding decoy molecules to hydroxylate unnatural substrates such as benzene and propane. We have succeeded in improving the hydroxylation efficiency of unnatural substrates by evolving the structure of the decoy molecules from the first generation to the third generation. Furthermore, we have succeeded in highly efficient direct hydroxylation of propane and ethane by developing a unique HPLC-type high-pressure reactor (Chapter 2, Episode 7: Development of a high-pressure reactor and highly efficient hydroxylation of gas molecules). However, we had not achieved direct hydroxylation of methane, the simplest hydrocarbon. Conversion of methane to methanol by catalytic direct hydroxylation is an effective reaction for utilizing methane hydrate, one of the few natural resources in Japan, as a chemical industrial raw material, and there has been a strong demand for the development of this technology. However, it is said to be one of the most difficult dream reactions among catalytic oxidation reactions. One of the reasons why direct hydroxylation of methane is difficult is that the C-H bond of methane is extremely stable (= poor reactivity. The dissociation energy of the C-H bond of methane is 104.9 kcal/mol, compared with 101.4 kcal/mol of ethane and 98.6 kcal/mol of the secondary carbon of propane). In addition, the minimum molecular size of methane is a significant bottleneck in the hydroxylation of methane by enzymes, leading to the difficulty of constructing an enzyme-methane complex, in other words, the difficulty of placing a methane molecule at the appropriate position within the enzyme. For this reason, methane hydroxylation was not observed even if a decoy molecule was used to control the reaction space near the active center of P450BM3. In addition, no P450 capable of hydroxylating methane has yet been found in nature.
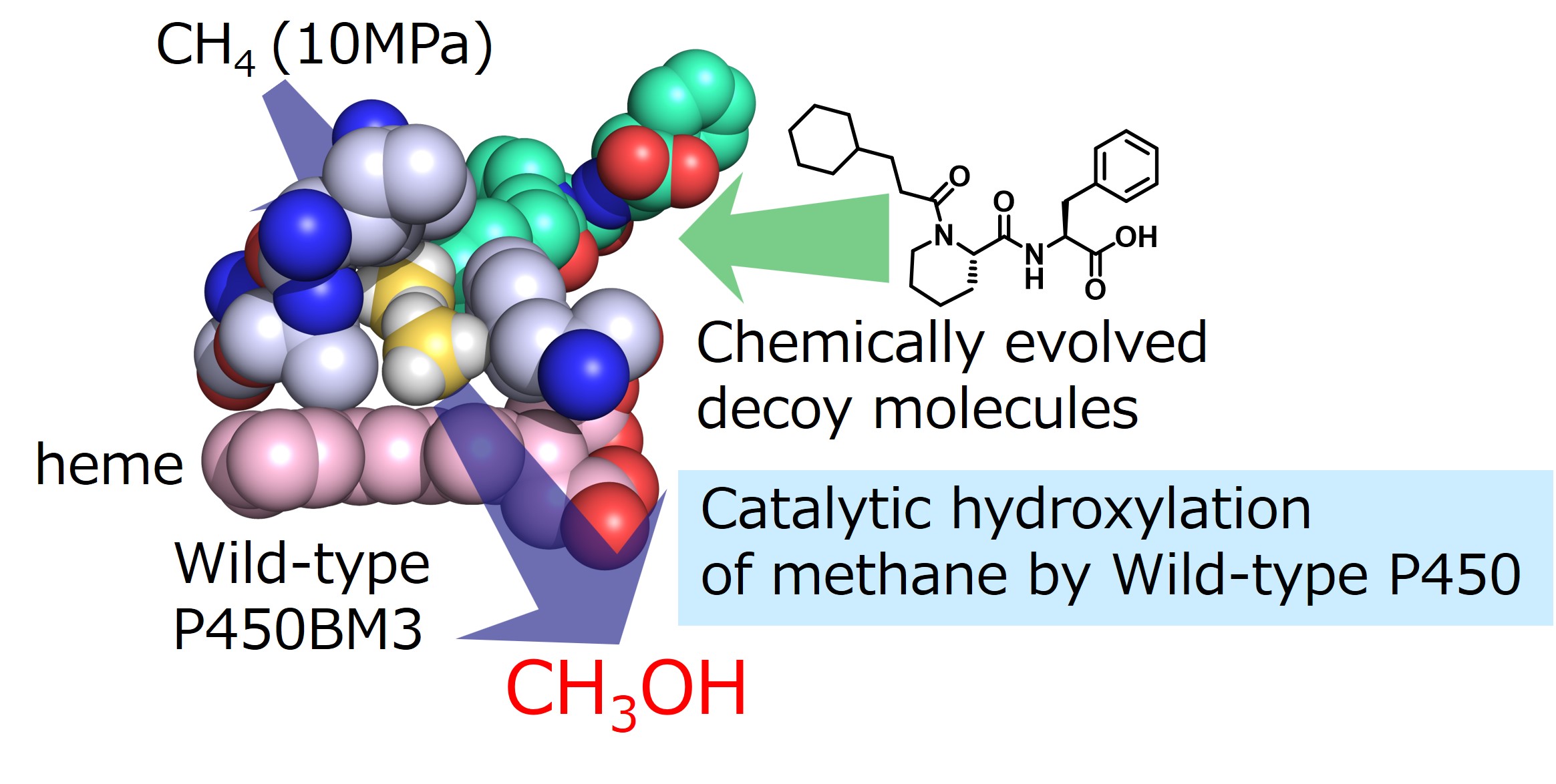
To address the challenging task of direct hydroxylation of methane using P450, we optimized the structure of the decoy molecule for methane hydroxylation and reconstructed the active center of P450BM3 for methane. We used a library of dipeptide-type decoy molecules previously created during the chemical evolution of decoy molecules (Episode 9: Chemical evolution of decoy molecules by multi-step screening) and used bromomethane, which can be screened by color reaction as a surrogate substrate instead of methane, and selected six candidates of decoy molecules that are expected to be suitable for methane hydroxylation using a microplate reader. Furthermore, in the hydroxylation of methane, the amount of methanol produced as a product is not very large due to its low reactivity. Therefore, in order to accurately estimate the amount of methanol produced by the enzymatic reaction of P450BM3, we used 13C-labeled methane gas as a substrate and further utilized sample concentration by solid-phase microextraction (SPME) for GCMS analysis. The decoy molecule candidates selected by screening were added to wild-type P450BM3 without mutations, and the enzymatic reaction was carried out under 13C-methane at 10 MPa in a high-pressure reactor. A peak derived from 13C-labeled methanol was clearly observed in the GCMS analysis. From the crystal structure analysis, it is predicted that the chemical evolution decoy molecule found for methane hydroxylation covers the periphery of the heme of P450BM3 and plays a role in preventing the methane molecule taken up from escaping from the vicinity of the heme. The efficiency of catalytic hydroxylation of methane by P450BM3 using the decoy molecule in this study was only four turns per enzyme per hour, which is not practical. However, the result of eliciting the ability to hydroxylate methane, the most difficult hydroxylation reaction, by simply adding a chemically synthesized decoy molecule to natural P450BM3 that exists in nature shows the high potential of P450 and also indicates the possibility that the substrate misrecognition system using the decoy molecule can give natural enzymes abilities that exceed those of nature.
Please refer to this paper for details.
- S. Ariyasu, K. Yonemura, C. Kasai, Y. Aiba, H. Onoda, Y. Shisaka, H. Sugimoto, T. Tosha, M. Kubo, T. Kamachi, K. Yoshizawa, O. Shoji, "Catalytic Oxidation of Methane by Wild-Type Cytochrome P450BM3 with Chemically Evolved Decoy Molecules" , ACS Catal., 13, (2023) 8613-8623
https://doi.org/10.1021/acscatal.3c01158