Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Chapter 2 Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
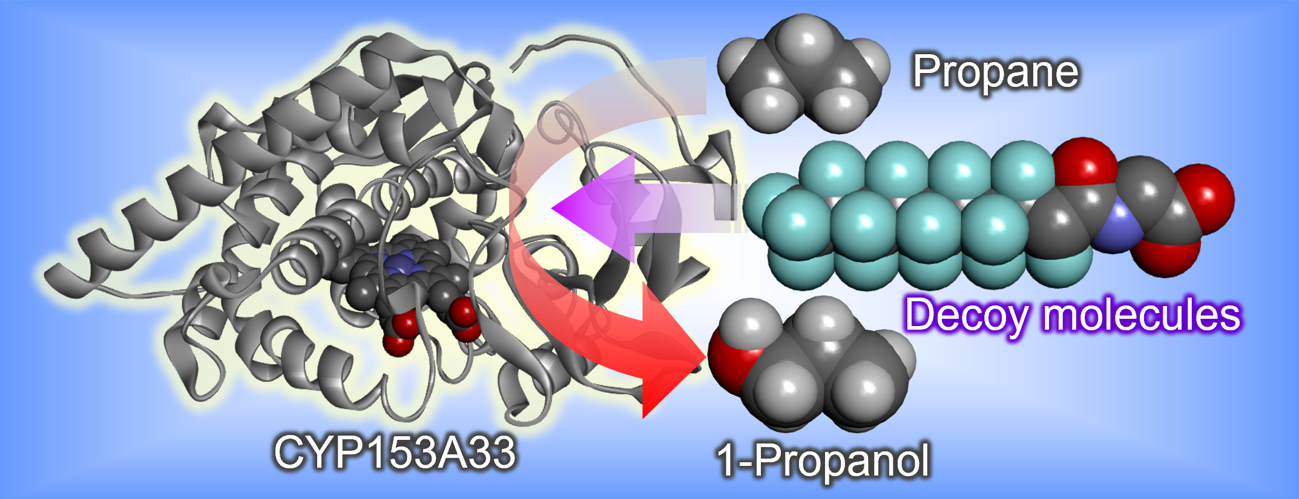
Episode 16: Terminal-selective hydroxylation of propane by substrate misrecognition using long-chain fatty acid terminal hydroxylase CYP153A
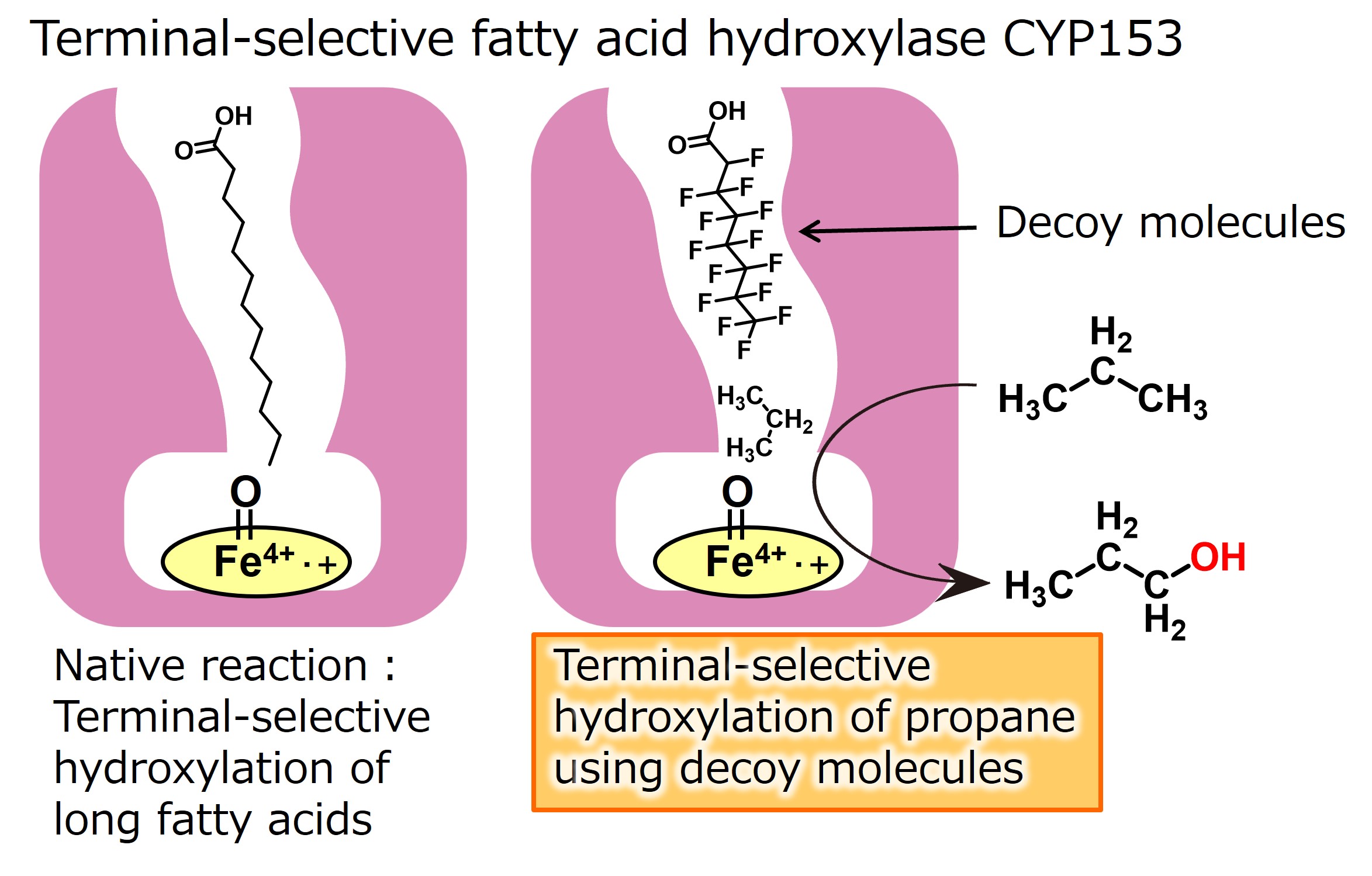
We have previously succeeded in directly hydroxylating propane (non-native substrate) by adding decoy molecules to the long-chain fatty acid hydroxylase P450BM3 derived from Bacillus megaterium. In the hydroxylation of propane, 1-propanol hydroxylated at the terminal primary carbon and 2-propanol hydroxylated at the central secondary carbon can be produced. Still, the substrate misrecognition system of P450BM3 produces 2-propanol with approximately 95% selectivity. This selectivity was almost unchanged for the many decoy molecules developed, even under high-pressure reaction conditions. The hydroxylation efficiency was improved, but the selectivity was virtually unchanged. This is related to the fact that in the hydroxylation of C-H bonds by P450, the dissociation of the C-H bond is considered to be the rate-limiting step, and the stability of the C-H bond strongly affects the reaction selectivity. In the case of propane, the C-H bond at the central secondary carbon is more reactive. In addition, because the space around the heme of P450BM3 has a relatively large space, and even in the hydroxylation of long-chain fatty acids, the hydroxylation of the less reactive terminal does not proceed, and the hydroxylation of the internal secondary carbon is prioritized. Reflecting this, even if a decoy molecule partially occupies the reaction space, it is not easy to completely fix the orientation of the propane. Therefore, it is thought that 2-propanol is preferentially obtained in P450BM3, and it is challenging to produce 1-propanol by selective terminal hydroxylation of propane.
Therefore, it would be challenging to achieve terminal-selective hydroxylation of propane using P450BM3 and we planned to use another P450 suitable for terminal-selective hydroxylation. We focused on CYP153, which selectively hydroxylates the terminals of long-chain fatty acids in nature. Compared to P450BM3, this enzyme group has a long, narrow tunnel-like substrate pocket, allowing only the terminals of long-chain fatty acids to approach the heme. This prevents hydroxylation of the highly reactive internal secondary carbons and enables highly selective hydroxylation of the terminal primary carbons. We speculated that terminal-selective hydroxylation of propane could be achieved by effectively utilizing the characteristic tunnel-like pocket of CYP153 and applying a substrate misrecognition system using a decoy molecule. Therefore, we selected CYP153A33 from the CYP153 subfamily and expressed it in E. coli. CYP153 is not a self-sufficient P450 that has reductases in the molecule, such as P450BM3, and therefore requires two reductases as reduction partners. When a solution containing CYP153 and two reductases was added with the decoy molecule previously developed for P450BM3 and a propane hydroxylation reaction was performed, the terminal hydroxylation product 1-propanol was produced with approximately 80% selectivity as expected, and it became possible to produce 2-propanol and 1-propanol by simply using different enzymes, P450BM3 and CYP153A33. This result not only shows that the substrate misrecognition system using the decoy molecule is effective for P450s other than the CYP102 subfamily, including P450BM3, but also suggests that various chemical substances can be produced by utilizing the structure of the substrate pocket of each P450.
Please refer to this paper for details.
- Y. Kodama, S. Ariyasu, M. Karasawa, Y. Aiba, O. Shoji, "Highly Selective Hydroxylation of Gaseous Alkanes at the Terminal Position by Wild-type CYP153A33" , Catal. Sci. Technol., 13, (2023) 6146-6152
https://doi.org/10.1039/D3CY00752A