Research Introduction
Artificial metalloproteins based on heme acquisition protein HasA
Episode 6: Conjugation of tetraphenylporphyrin complexes with HasA and catalytic reactions using protein crystals
Previously, we have succeeded in conjugating HasA with diphenylporphyrin (DPP) and triphenylporphyrin (TriPP) but have not succeeded in stably conjugating a metal complex of tetraphenylporphyrin (TPP), which has four phenyl groups, to HasA. TPP is a porphyrin skeleton first chemically synthesized by Rothemund in 1935. Many derivatives have been synthesized as the most versatile porphyrin skeleton, but no examples of stable conjugation of TPP to proteins were reported.
In this context, we have investigated the conditions for complexing the TPP iron complex with HasA and found that FeTPP-HasA can be stably complexed under alkaline conditions. This achievement led to the complexation of HasA with TPP complexes of chromium, manganese, cobalt, and gallium in addition to iron, and also to the complexation of the TPyP complex, which has pyridyl groups instead of the four phenyl groups of TPP, and the TFFFP complex, which has a pentafluorophenyl group, by combining with the introduction of mutations into HasA.
The TPP-HasA conjugates showed a growth inhibitory effect against Pseudomonas aeruginosa, and we found that CoTPP-HasA, which has a cobalt central metal, showed a potent inhibitory effect. We also succeeded in photosterilization of Pseudomonas aeruginosa by irradiating it with 420 nm light using GaTPP-HasA.
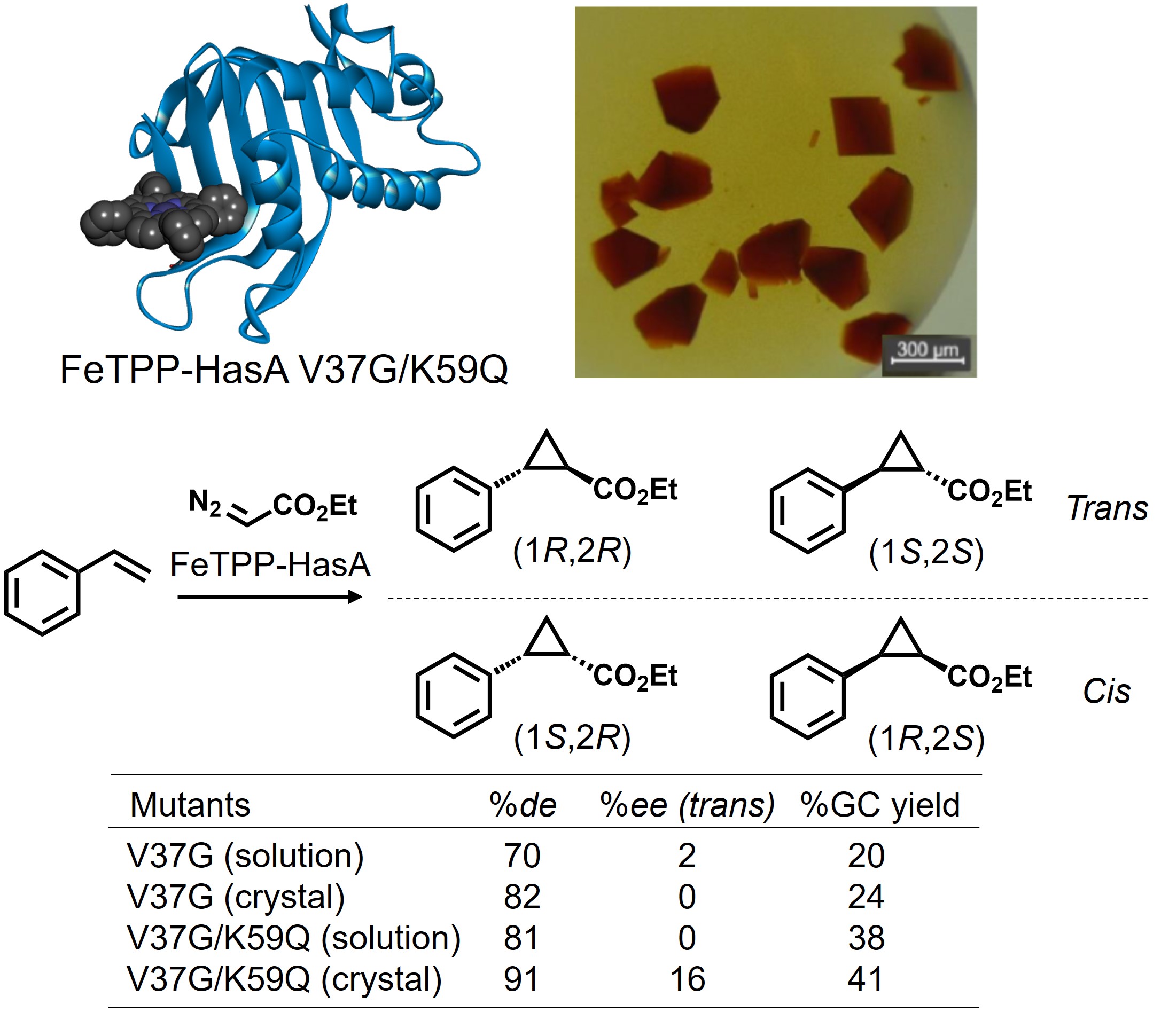
Furthermore, we analyzed the crystal structure of a complex between a HasA double mutant (V37G/K59Q) and FeTPP. We found that the steric hindrance of the phenyl group of TPP caused the loop structure of HasA to change. The coordination of the His residue, one of the two coordination amino acids of HasA that fix the metal complex, was flipped, resulting in a five-coordinate structure around FeTPP. In addition, a polymer-like packing structure was shown by fitting a loop from another HasA molecule into the space created in FeTPP. We used this characteristic crystal structure as an artificial reaction space to perform cyclopropanation of styrene and showed stereoselectivity, indicating the potential application of artificial HasA to catalysis.
Please refer to this paper for details.
- Y. Shisaka, E. Sakakibara, K. Suzuki, J. K. Stanfield, H. Onoda, G. Ueda, M. Hatano, H. Sugimoto, O. Shoji "Tetraphenylporphyrin Enters the Ring: First Example of a Complex Between Highly Bulky Porphyrins and a Protein" , ChemBioChem, 23, e202200095 (2023)
https://doi.org/10.1002/cbic.202200095