Research Introduction
Gene regulation by peptide nucleic acid (PNA)
All proteins that control various biological functions in the body are coded as DNA information. Within cells, only the necessary parts of DNA are copied (transcribed) to mRNA, which is then used to produce proteins (translation). Since many diseases are caused by proteins, such as overexpression or insufficient expression, developing drugs for proteins as therapeutic targets has progressed. In recent years, the deciphering of the human genome has led to progress in research into nucleic acid medicines that target DNA and RNA.
DNA and RNA are biopolymers that have four nucleic acid bases: adenine (A), thymine (T) (uracil (U) in the case of RNA), cytosine (C), and guanine (G). They can form A-T and G-C pairs through hydrogen bonds and, therefore, have the characteristic of forming double strands with complementary strands that have paired nucleic acid bases. In nucleic acid drugs, based on the base sequence information of DNA and RNA, artificial nucleic acids with complementary base sequences are added to form double strands at specific positions, aiming to control the expression of proteins related to diseases and treat them. To be used in nucleic acid drugs, artificial nucleic acids must have many properties, such as stability in the body, strong binding to the target base sequence, and the ability to not bind to sites other than the target sequence (mismatch recognition ability). Many researchers worldwide are developing various artificial nucleic acids in search of better artificial nucleic acids.
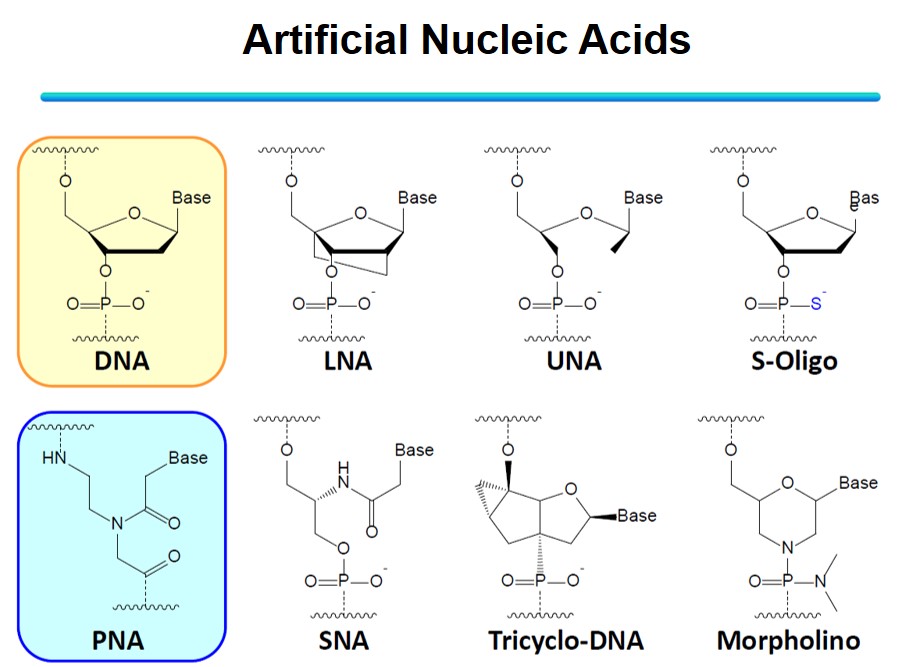
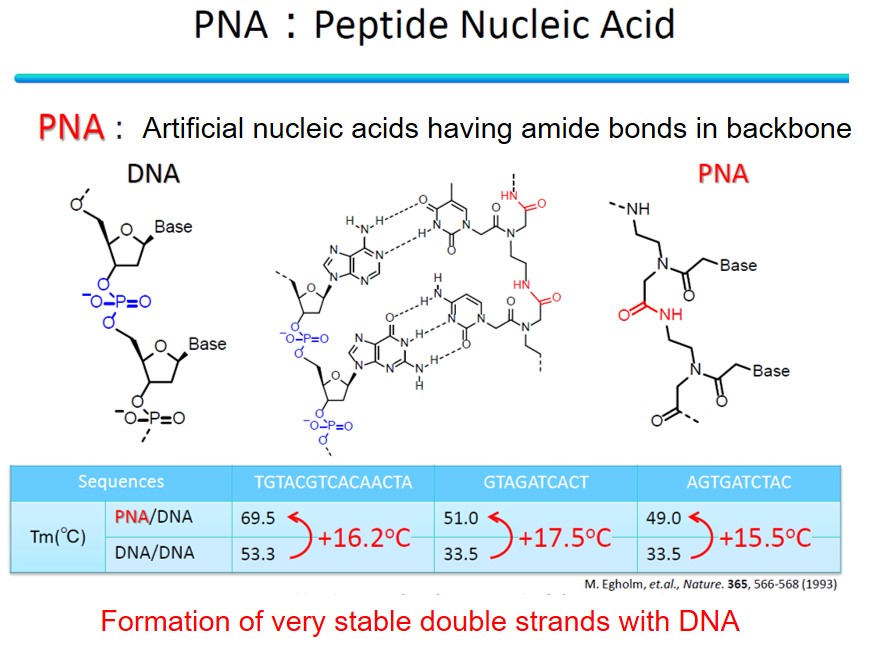
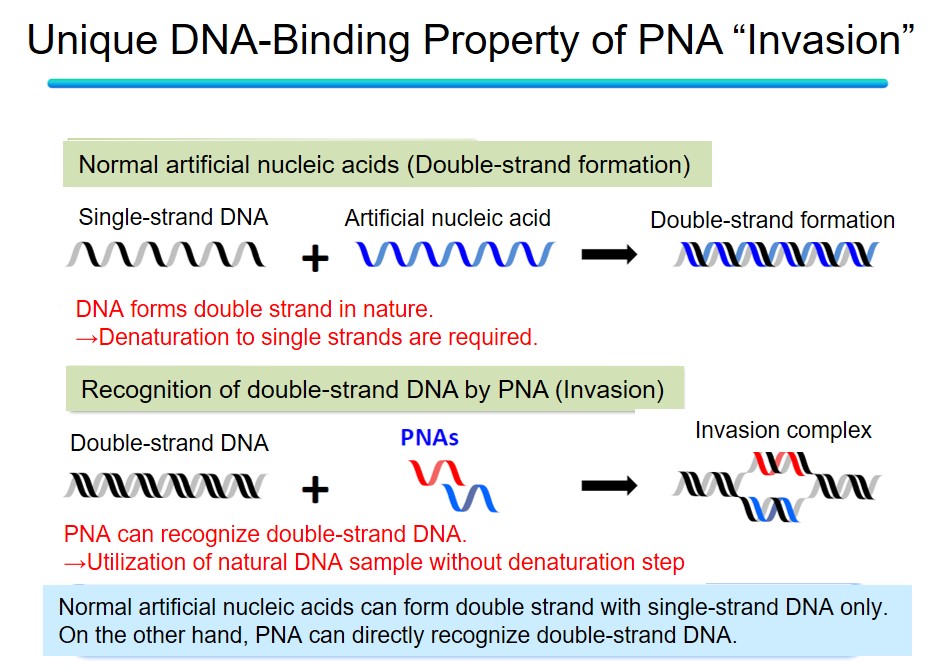
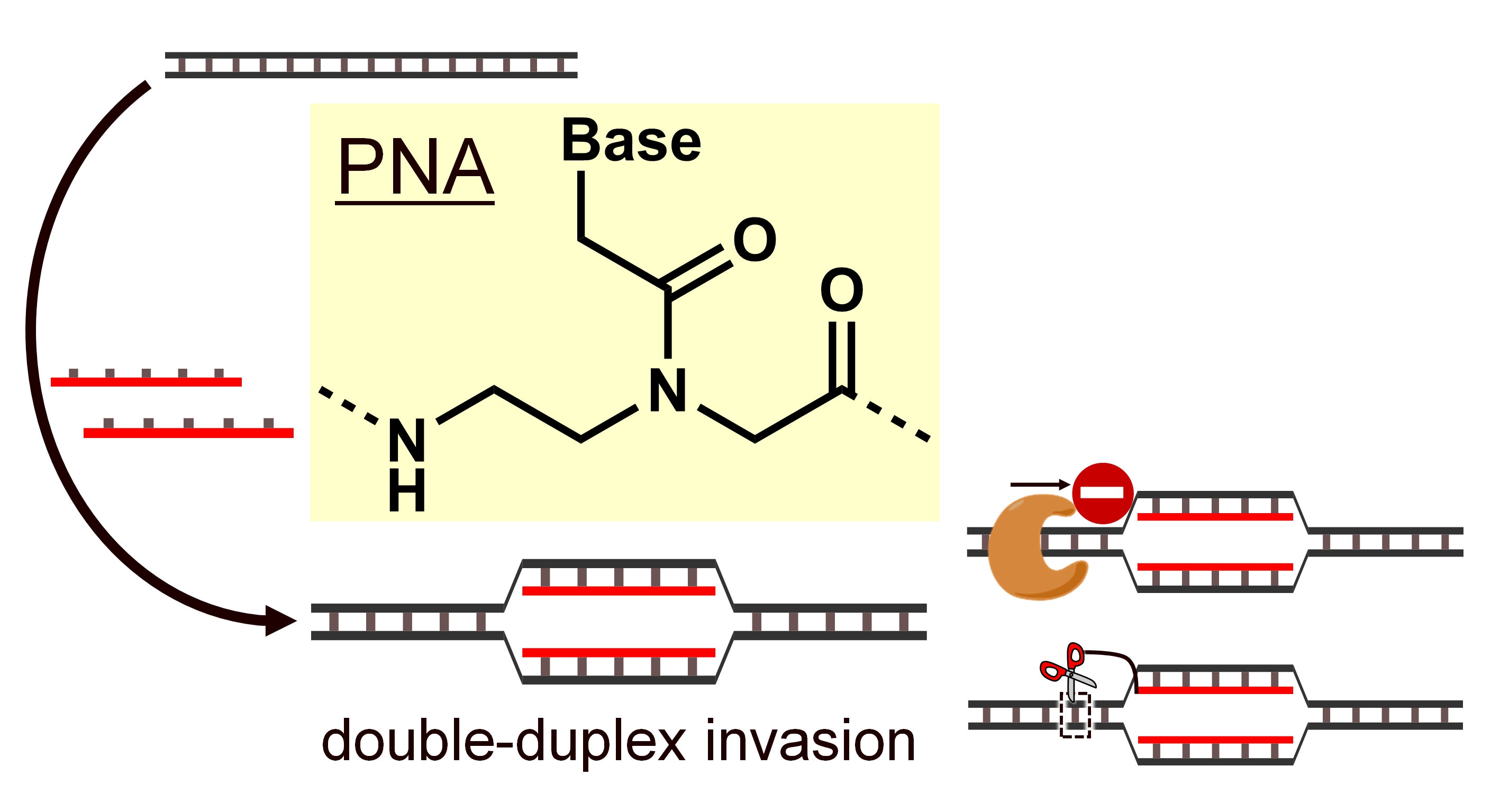
Among them, we are focusing on peptide nucleic acid (PNA). PNA is an artificial nucleic acid with an amide bond as the backbone. A phosphate diester backbone in DNA has a negative charge, but PNA is uncharged. Typically, electrostatic repulsion occurs between the negative charges of the two DNA strands when forming a DNA-DNA double strand. However, since PNA has no charge, it can form a stable double strand with the target DNA without electrostatic repulsion. Furthermore, PNA can perform a unique DNA binding mode called "invasion" that other artificial nucleic acids do not achieve. Because DNA forms a stable double helix structure in the body, it is necessary to denature the natural DNA to make it a single strand and form a double strand between a target DNA and a general artificial nucleic acid. Breaking duplex DNA requires high-temperature conditions, an obstacle to its application in the body. On the other hand, PNA can bind to the duplex structure of DNA itself in an invasive manner and form an invasion complex without denaturing processes, which is expected to be applied in the body, such as nucleic acid medicine.
The latest PNA research is explained in the review.
- Y. Aiba, M. Shibata, O. Shoji "Sequence-Specific Recognition of Double-Stranded DNA by Peptide Nucleic Acid Forming Double-Duplex Invasion Complex" , Appl. Sci., 12, (2022) 3677.
https://doi.org/10.3390/app12073677
Episode 1: Improvement of PNA invasion efficiency by conjugation with ruthenium complexes
Episode 2: Cationic guanine-introduced PNA (masaPNA) by methylation of guanine
Episode 3: Improved invasion efficiency of PNA by linking nuclear localization signal peptide (NLS)
Episode 4: Highly efficient invasion of mismatched PNA into mismatched sites of double-stranded DNA


