Research Introduction
Artificial metalloproteins based on heme acquisition protein HasA
Pseudomonas aeruginosa is a resident bacterium that is widely distributed around the body and is usually not pathogenic, but is classified as an opportunistic infectious bacterium that is pathogenic only to people with reduced immunity. P. aeruginosa produces many virulence factors, causing pneumonia, sepsis, etc., and is particularly problematic for hospital infections. Recently, due to inappropriate use of antibiotics (antibacterial agents), the emergence of multidrug-resistant P. aeruginosa having resistance to multiple antibiotics has been regarded as a problem. P. aeruginosa has a high speed of acquisition of drug resistance, and even if a new antibacterial agent is developed, P. aeruginosa can acquire resistance ability. Therefore, there is a big problem that treatment using the antibacterial agent is difficult. Therefore, sterilization methods with mechanisms different from that of antibacterial agents are strongly demanded all over the world.
P. aeruginosa also requires iron for growth. Therefore, various mechanisms to acquire iron from the environment have been developed. One of them is called the heme acquisition system (Has), which takes up heme (an iron complex) from heme proteins (eg, hemoglobin) held by hosts such as humans and is used as an iron source. This system is usually used in an environment where the iron source is insufficient.
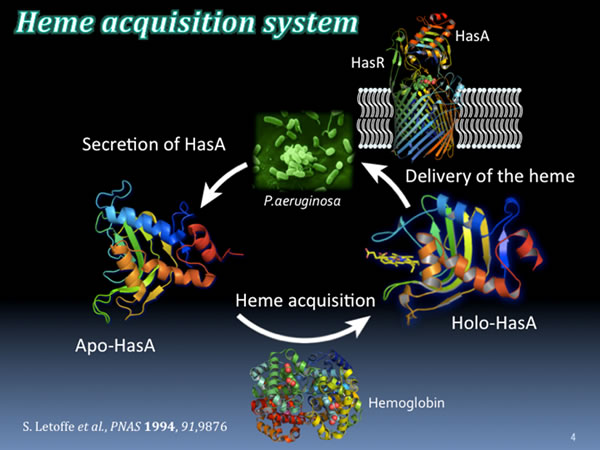
The first stage of the heme acquisition mechanism begins with P. aeruginosa secreting a small protein called apo-HasA (without heme). An apo-HasA has a strong binding affinity with heme, deprives heme from the host heme protein, and becomes helo-HasA with heme bound. Subsequently, heme is fed into P. aeruginosa using the HasA-specific receptor, HasR, present on the surface of P. aeruginosa and used as an iron source. This heme acquisition system is a system essential for the growth of P. aeruginosa. If this system is stopped, P. aeruginosa cannot secure the necessary iron source and their growth stops.
Episode 1: Conjugation of synthetic metal complexes to the heme acquisition protein HasA and growth inhibition of Pseudomonas aeruginosa
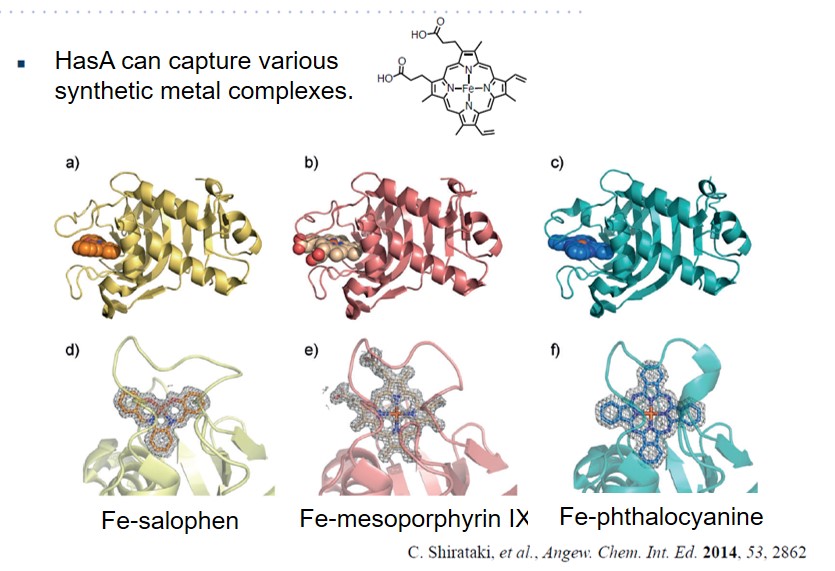
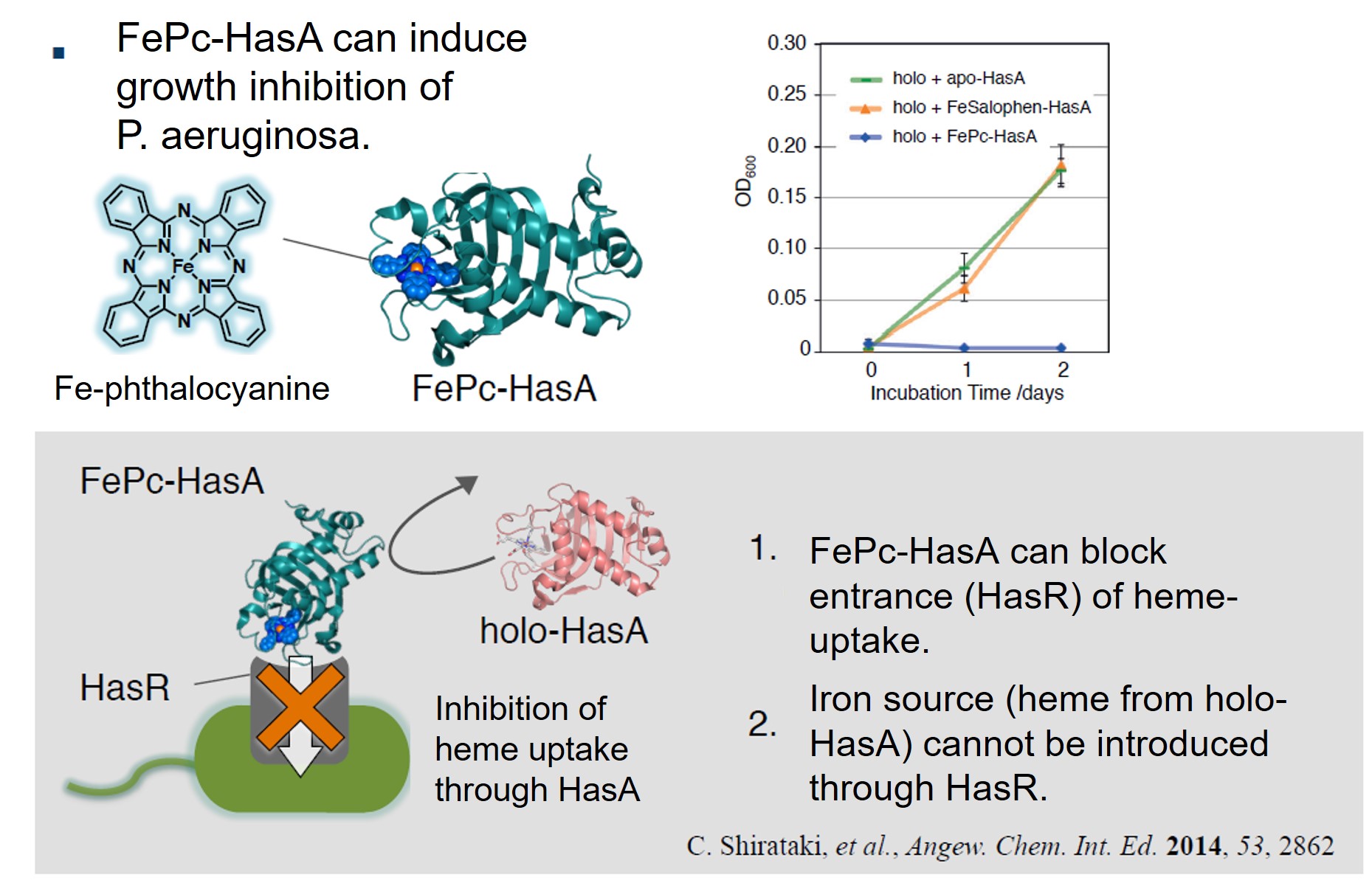
HasA not only has excellent binding affinity to heme, but also has a high resistance to organic solvents, and also has a unique property that the heme binding site is exposed to the outside. Using these properties, we succeeded in complexation of various synthetic metal complexes other than natural heme with apo-HasA. Synthetic complexes that can be captured by HasA are not only porphyrins that have been extensively studied as heme model complexes, but also synthetic complexes with a structure different from heme, such as phthalocyanine, which has a larger plain structure than heme, can be complexed with HasA. HasA can bind with iron phthalocyanine (FePc) that is an artificial synthetic metal complex that does not exist in nature. Interestingly, the protein structure of HasA in FePc-HasA conjugate is almost the same as holo-HasA bound with natural heme. In agreement with holo-HasA, FePc-HasA can also bind to the dedicated receptor HasR of P. aeruginosa. Furthermore, when FePc-HasA was added to P. aeruginosa under iron restriction condition, growth inhibition of P. aeruginosa was observed. This is because FePc has a larger structure than natural heme, so FePc delivered from FePc-HasA to HasR cannot pass along the way, blocking the system of P. aeruginosa heme acquisition.
Please refer to this paper for details.
- C. Shirataki, O. Shoji, M. Terada, S. Ozaki, H. Sugimoto, Y. Shiro, Y. Watanabe, "Inhibition of Heme Uptake in Pseudomonas aeruginosa by its Hemophore (HasAp) Bound to Synthetic Metal Complexes" , Angew. Chem. Int. Ed., 53, 2862–2866 (2014). The inside cover of the issue 11.
http://dx.doi.org/10.1002/anie.201307889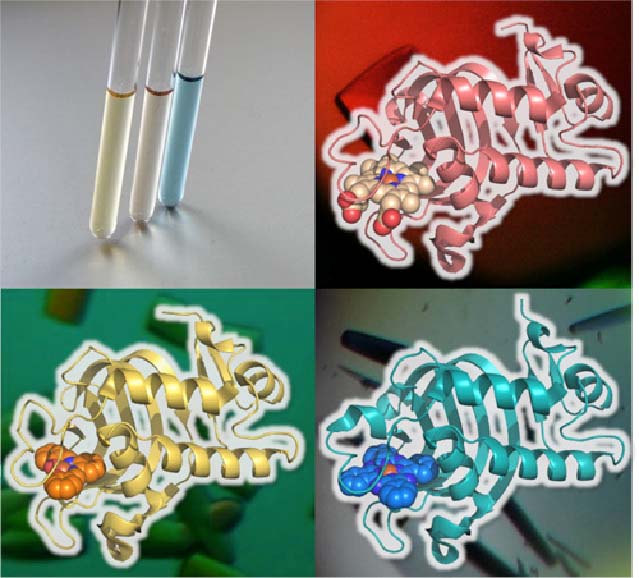
Episode 5: Complexation of Pseudomonas aeruginosa HasA with 5-oxaporphyrinium cation complex


