Research Introduction
Development of Biocatalysts Based on Cytochrome P450s
Cytochrome P450s and Decoy Molecules
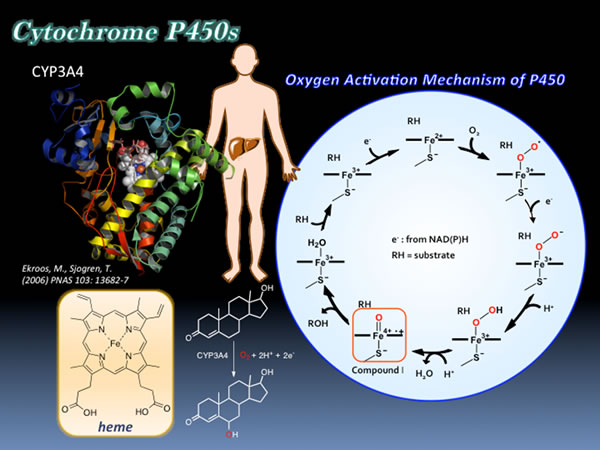
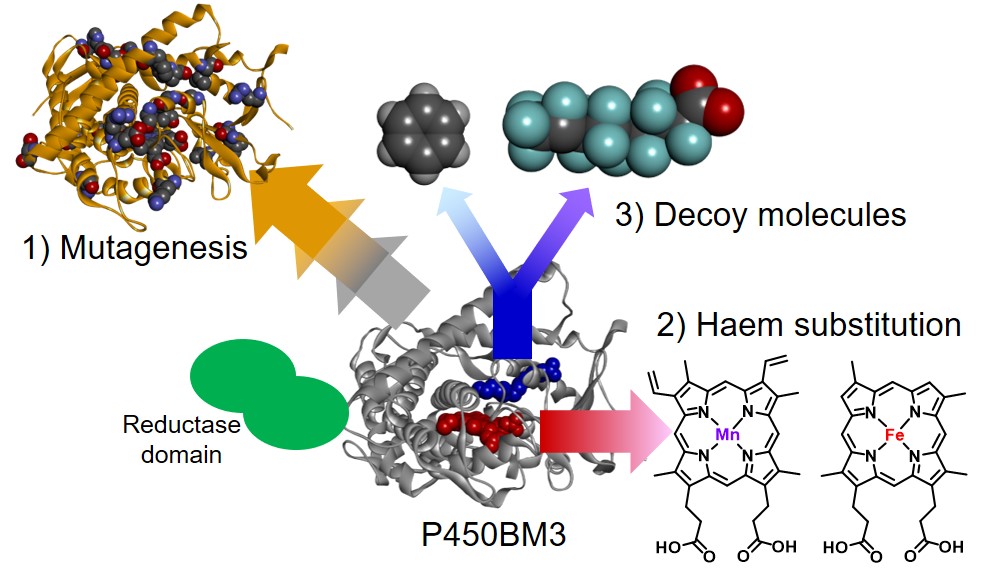
Cytochrome P450s (P450s) are ubiquitous enzymes that comprise a superfamily of heme-containing monooxygenases and involved in oxidative metabolism, detoxification, and synthesis of steroids. P450s have been regarded as attractive candidates for oxidation catalysts because of their high catalytic activity for direct oxygen insertion into unactivated C-H bonds. In general, bacterial cytochrome P450s (P450s) possess high catalytic activity and high substrate specificity at the same time. To utilize bacterial P450s as a biocatalyst for the oxidation of various substrates, we have to alter their substrate specificity. Although site-directed and random mutagenesis have been regarded as one of the most powerful approach to change the substrate specificity of P450s, we recently have demonstrated that the substrate specificity of hydrogen peroxide-dependent P450s (P450BSβ and P450SPα) can be altered just by adding a decoy molecule, which has a structural similarity to their natural substrate. In this reaction system, the decoy molecule induced substrate misrecognition and non-natural substrates were oxidized by P450BSβ and P450SPα without replacing any amino acid residues. More recently, we have expanded this substrate misrecognition system to include P450BM3. Utilizing perfluorinated carboxylic acids as a decoy molecule toward P450BM3, we have succeeded in hydroxylation of propane and butane. Interestingly, the catalytic activity of P450BM3 are dependent on the alkyl chain length of perfluorinated carboxylic acid and perfluorinated decanoic acid gave the highest rate of product formation for propane hydroxylation among perfluorinated carboxylic acids examined. These results indicate that the substrate specificity can be changed by adding decoy molecules and the catalytic activity can be controlled by the choice of decoy molecule.
Chapter 1: Functional modulation of hydrogen peroxide-driven P450 using decoy molecules
Chapter 2: Functional modulation of P450BM3 using decoy molecule and its application to biocatalyst
Chapter 2 Episode 4: Control of stereoselectivity in hydroxylation by decoy molecules
Chapter 2 Episode 5: Reconstitution of P450BM3 for incorporation of different metal centers
Chapter 2 Episode 6: Direct conversion from benzene to phenol using E. coli as a reaction vessel
Chapter 2 Episode 8: Rapid crystallization of P450BM3 by crystallization-inducing decoy molecule
Chapter 2 Episode 9: Systematic evolution of decoy molecules through multi-step screening
Chapter 2 Episode 14: Application of decoy molecules to CYP102A5 and A7 in subfamily of P450BM3
Please refer to this paper for details about alteration of P450's function.
- S. Ariyasu, J. K. Stanfield, Y. Aiba, O. Shoji "Expanding the Applicability of Cytochrome P450s and other Haemoproteins" , Curr. Opin, Chem. Biol., 59,(2020) 155-163
https://doi.org/10.1016/j.cbpa.2020.06.010